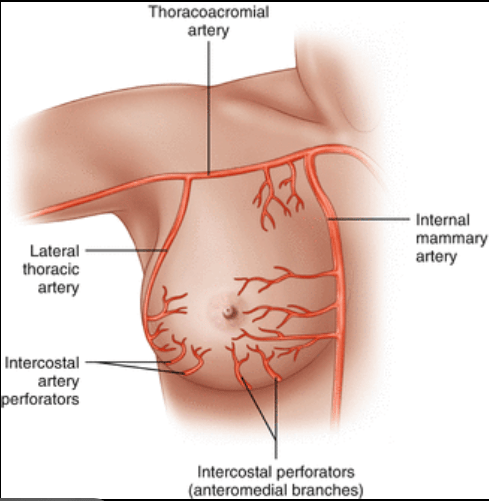
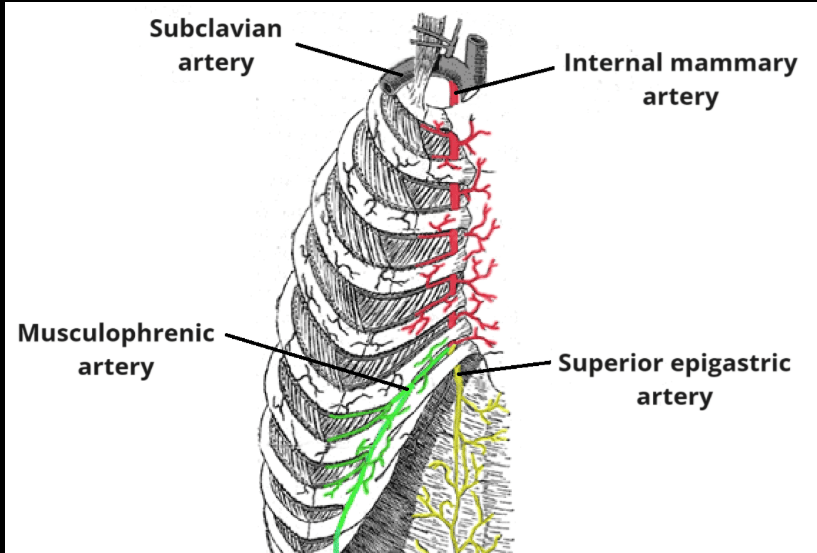
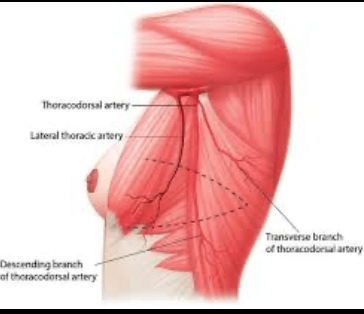
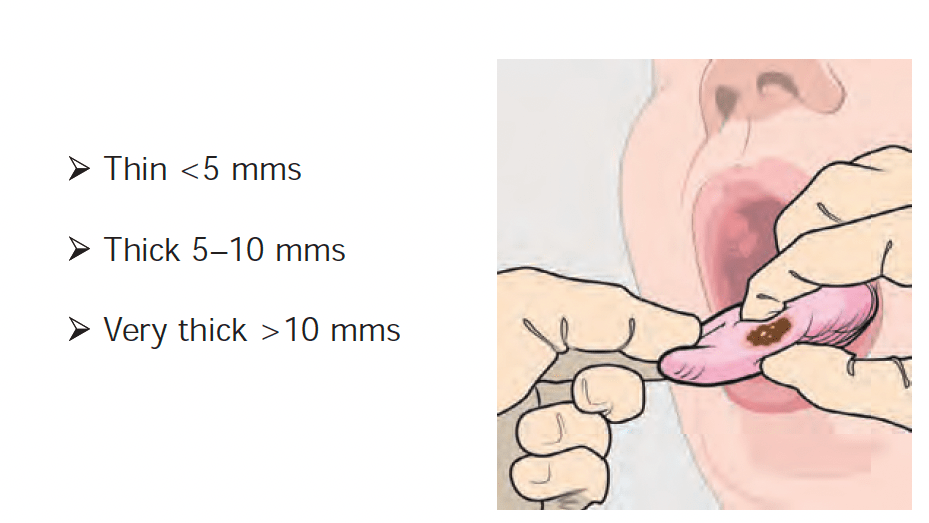
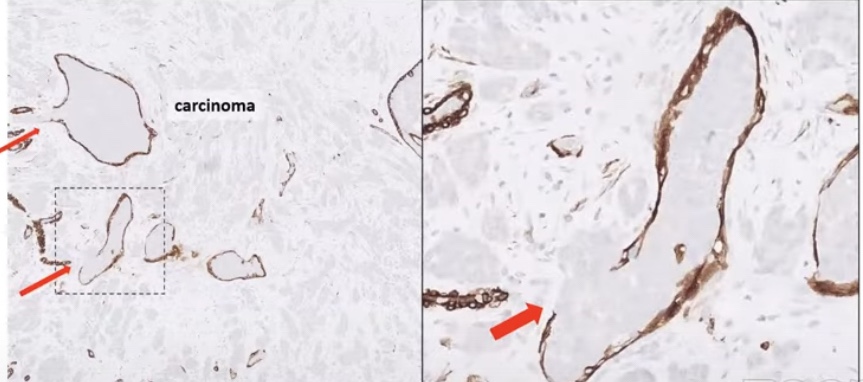
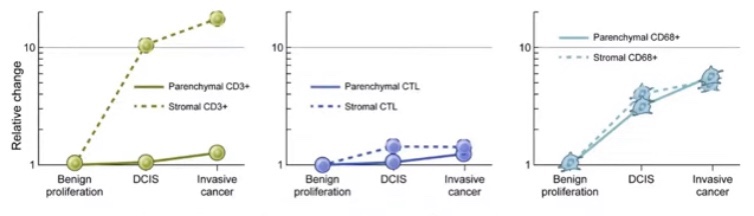
- What DOI is (and why it matters):
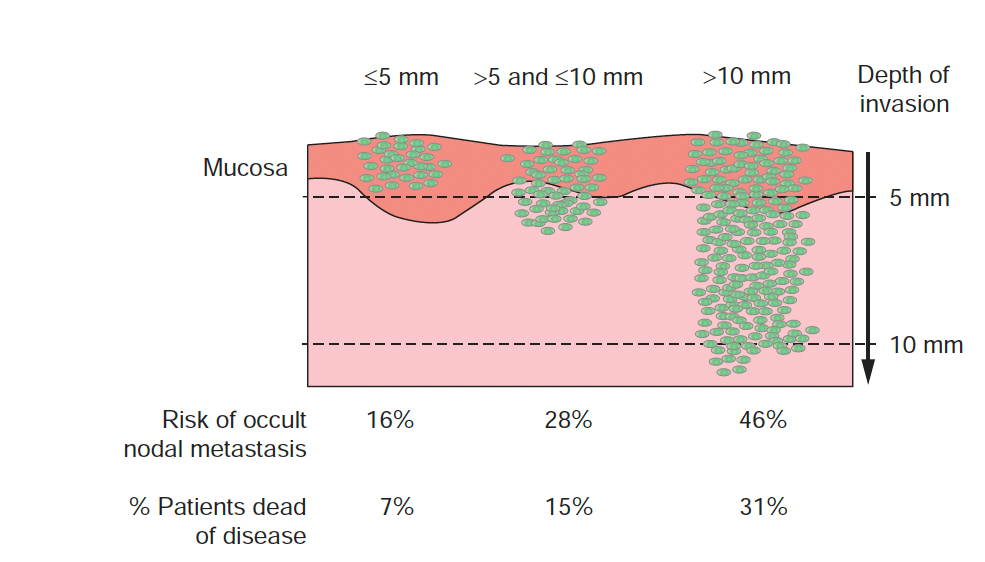
- Depth of invasion (DOI) is the vertical depth of tumor invasion:
- Measured from the basement membrane of the adjacent normal mucosa to the deepest point of invasion:
- It is not the same as “tumor thickness”
- Measured from the basement membrane of the adjacent normal mucosa to the deepest point of invasion:
- DOI is now a core determinant of T category in AJCC 8 for oral cavity SCC:
- AJCC 8 DOI cut points (oral cavity):
- T1:
- ≤ 2 cm and DOI ≤ 5 mm
- T2:
- ≤ 2 cm with DOI > 5 to 10 mm OR > 2 to 4 cm with DOI ≤ 10 mm
- T3:
- DOI >10 mm (or tumor > 4 cm)
- T1:
- AJCC 8 DOI cut points (oral cavity):
- Clinical implication:
- A small “T1 by size” lesion can become T2 / T3 purely based on DOI:
- Changing risk counseling and neck strategy
- A small “T1 by size” lesion can become T2 / T3 purely based on DOI:
- Depth of invasion (DOI) is the vertical depth of tumor invasion:
- Risk of occult nodal metastasis vs DOI (tongue and floor of mouth):
- Big picture (consistent across studies):
- DOI is one of the strongest predictors of occult cervical lymph node metastasis (CLNM) in cN0 oral cavity SCC
- A commonly used operative decision threshold is DOI ~ 3 to 4 mm:
- But subsite matters, and FOM often carries higher nodal risk at the same DOI
- Big picture (consistent across studies):
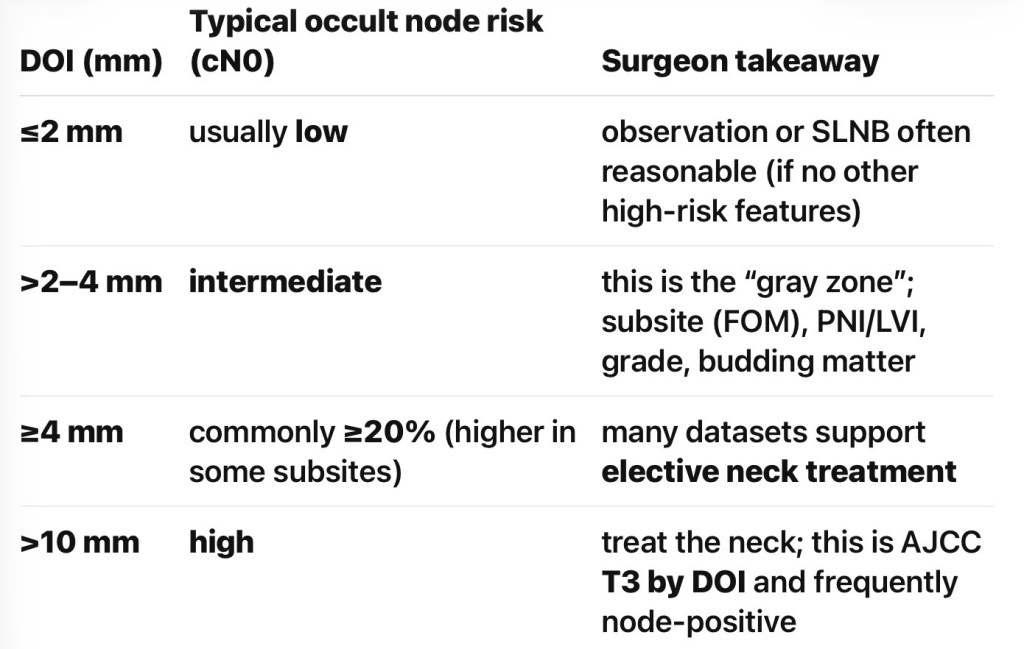
Practical DOI “risk bands” used in tumor boards
- Evidence supporting ≥ 4 mm as an elective neck dissection (END) trigger (early OCSCC):
- Multiple analyses suggest DOI ≥ 4 mm is an effective cutoff where END improves regional control / survival compared with observation in early-stage OCSCC
- Recent work continues to evaluate / validate a 4 mm threshold, acknowledging imperfect sensitivity / specificity
- Meta-analytic evidence shows higher lymph node metastasis (LNM) risk when DOI > 4 mm (RR ~2.18 in one large study, alongside other adverse pathologic factors)
- Floor of mouth nuance:
- At the same DOI:
- FOM cancers may metastasize more frequently than tongue cancers in some datasets:
- Implying that a single universal DOI cutoff across all subsites can be overly simplistic
- FOM cancers may metastasize more frequently than tongue cancers in some datasets:
- At the same DOI:
- Prognosis vs DOI (local control, survival, and upstaging):
- DOI correlates with:
- Higher probability of nodal metastasis:
- Including occult disease
- Worse disease-specific outcomes:
- It is sufficiently prognostic that it was incorporated into AJCC 8 edition T staging
- DOI > 10 mm is particularly important because it upstages to pT3 (even if tumor is small in surface dimension):
- Reflecting its association with advanced behavior
- Higher probability of nodal metastasis:
- Key point for counseling:
- DOI is not just a “neck decision tool”:
- It is a global biologic aggressiveness marker and a staging variable
- DOI is not just a “neck decision tool”:
- DOI correlates with:
- Elective neck management in cN0 tongue / FOM SCC:
- Guideline-consistent approach:
- NCCN guidance (summarized in literature):
- Consider elective neck dissection (END) in early oral cavity SCC when DOI exceeds ~3 mm (often framed as “consider END”)
- Many institutions operationalize:
- Tongue:
- END commonly at ≥ 4 mm
- FOM:
- Lower threshold and / or stronger lean toward END due to higher nodal propensity in several series
- Tongue:
- NCCN guidance (summarized in literature):
- Guideline-consistent approach:
- END vs sentinel lymph node biopsy (SLNB) vs observation:
- Elective Neck Dissection (END):
- Typical for cN0 early tongue / FOM:
- Selective neck dissection levels I to III ± IV based on institutional practice, DOI, and risk factors:
- Benefit is maximizing regional control and avoiding “salvage neck failure” biology
- Selective neck dissection levels I to III ± IV based on institutional practice, DOI, and risk factors:
- Typical for cN0 early tongue / FOM:
- Sentinel Lymph Node Biopsy (SLNB):
- Valid alternative to END for T1 to T2 cN0 oral cavity SCC in experienced centers:
- Especially when trying to reduce morbidity
- Practical pearl:
- SLNB is most attractive when DOI is low / intermediate and imaging is negative:
- But your workflow must support reliable mapping / pathology
- SLNB is most attractive when DOI is low / intermediate and imaging is negative:
- Valid alternative to END for T1 to T2 cN0 oral cavity SCC in experienced centers:
- Observation:
- Reasonable primarily for very thin lesions (e.g., ≤ 2 mm) without other high-risk features and with reliable follow-up
- Remember:
- DOI cutoffs have imperfect test characteristics:
- A “thin” tumor can still metastasize
- DOI cutoffs have imperfect test characteristics:
- Elective Neck Dissection (END):
- A pragmatic surgeon algorithm (tongue + floor of mouth, cN0):
- Pre-op:
- High-quality exam + imaging
- Estimate DOI if possible:
- US / MRI can help in some settings
- If DOI likely > 10 mm (or bulky lesion):
- Treat the neck (END)
- If DOI 4 to 10 mm:
- Strong default to END (levels I to III) or SLNB if program is robust
- If DOI 2 to 4 mm:
- Individualized:
- Subsite matters – FOM pushes toward END; add PNI / LVI / grade / budding into decision
- Individualized:
- If DOI ≤ 2 mm:
- Consider observation vs SLNB:
- Depending on subsite / risk factors and follow-up reliability
- Depending on subsite / risk factors and follow-up reliability
- Consider observation vs SLNB:
- Pre-op: