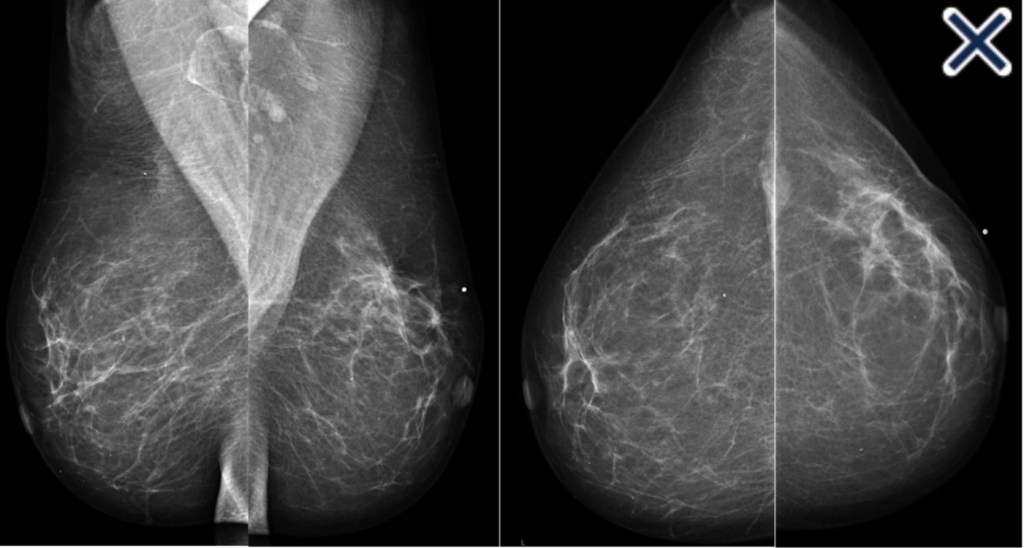
- Diffusely invasive carcinoma:
- Has a mammographic appearance of:
- Diffuse architectural distortion:
- Usually involving a large area, often larger than a lobe:
- With no central tumor mass and no calcifications
- Usually involving a large area, often larger than a lobe:
- Diffuse architectural distortion:
- It sometimes has the appearance of a “spider’s web” as shown in Image
- Has a mammographic appearance of:
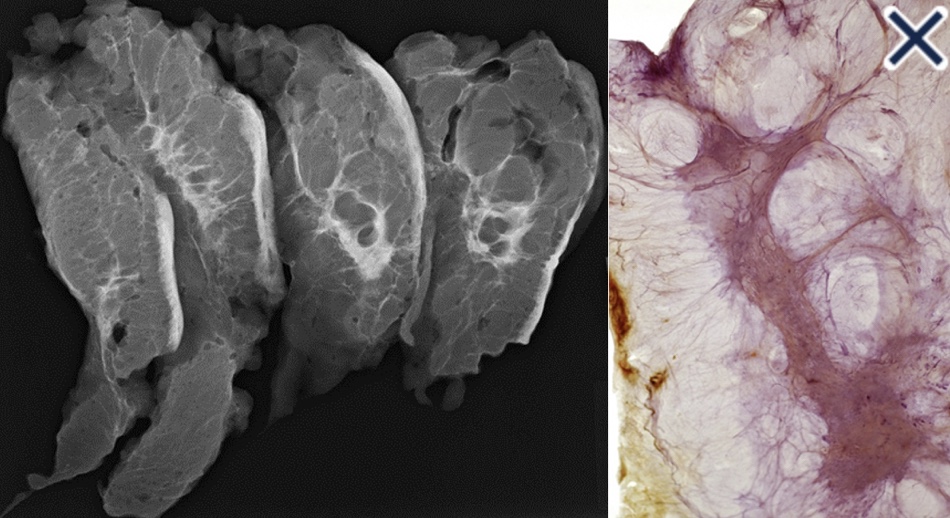
- The diffusely infiltrating cancer:
- Forms concave contours with the surrounding fat in a manner similar to normal fibroglandular tissue (Images Above)
- The imaging findings of diffusely infiltrating breast cancer:
- Are strikingly different from the imaging findings of breast cancers originating either from the terminal ductal lobular units (TDLUs) or the lactiferous ducts:
- Suggesting that it may have a different site of origin
- Are strikingly different from the imaging findings of breast cancers originating either from the terminal ductal lobular units (TDLUs) or the lactiferous ducts:
- It has been recently proposed that diffusely infiltrating breast cancers:
- May originate from mesenchymal stem cells (progenitors):
- Through a complex process of both:
- Epithelial-mesenchymal transformation and more frequently, mesenchymal-epithelial transformation
- Through a complex process of both:
- The clinical presentation is typically a:
- Recently detected, extensive, firm lesion:
- Often appearing as an interval cancer following a previous mammogram which was interpreted as normal
- On clinical breast examination:
- The cancer does not have a distinct tumor mass or focal skin retraction seen in other cancers:
- But rather an indistinct “thickening” and eventually a shrinkage of the breast
- In order to make the diagnosis before the development of a palpable mass and a decrease in size of the breast:
- The radiologist and breast surgeon must have a high level of suspicion and a thorough knowledge of the underlying pathophysiology
- The cancer does not have a distinct tumor mass or focal skin retraction seen in other cancers:
- Recently detected, extensive, firm lesion:
- May originate from mesenchymal stem cells (progenitors):
- The subgross (3D) histopathology images:
- Show how growth of the mesenchymal tissue distorts the normal, harmonious connective tissue framework:
- By causing nonuniform thickening of the fine sheets of connective tissue
- Show how growth of the mesenchymal tissue distorts the normal, harmonious connective tissue framework:
- The predominance of mesenchyme in the diffusely infiltrating breast malignancy:
- Allows it to be imaged with greater sensitivity by ultrasound than by mammography
- The thin sheets or veils of tissue reflect the ultrasound waves:
- But are relatively easily penetrated by x-rays
- The structural / architectural distortion, while difficult to detect mammographically:
- Is readily detectable on 2-mm thick coronal sections of automated breast ultrasound
- The hypoechoic changes:
- Can also usually be seen on hand-held ultrasound
- The growth pattern and cell type of diffusely invasive breast cancer is very similar to that of diffuse gastric carcinoma (linitis plastica):
- Both of these diseases can be associated with a deleterious mutation in the CDH1 gene:
- Which is located on chromosome 16q22 and codes for e-cadherin protein
- Both of these diseases can be associated with a deleterious mutation in the CDH1 gene:
- References:
- Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23-32.
- Tot T. The diffuse type of invasive lobular carcinoma of the breast: morphology and prognosis. Virchows Arch. 2003;443(6):718-724.
- Tot T. Diffuse invasive breast carcinoma of no special type. Virchows Arch. 2016;468(2):199-206.