- Generalities:
- Warthin tumor (WT) is the second most common benign parotid gland tumor:
- After pleomorphic adenoma
- Almost exclusively arises in the parotid gland:
- Classically in the tail (inferior pole)
- Distinctive for its strong association with smoking and for being bilateral or multifocal:
- More often than any other salivary gland neoplasm
- Malignant transformation:
- Is exceedingly rare (< 1%)
- Growth is usually slow and indolent:
- Many tumors are discovered incidentally on imaging
- Warthin tumor (WT) is the second most common benign parotid gland tumor:
- Epidemiology:
- Accounts for 5% to 15% of all parotid tumors
- Account for 10% to 30% of benign parotid neoplasms, depending on population
- Accounts for 5% to 15% of all parotid tumors
- Peak incidence:
- 6th to 7th decades of life
- Historically showed strong male predominance (≈ 5:1):
- Now closer to 1.5 to 2:1:
- Reflecting increased smoking prevalence among women
- Now closer to 1.5 to 2:1:
- Bilateral tumors:
- ~ 7% to 10%
- Multifocal within the same gland:
- Up to 12% to 20%
- Risk Factors
- Established:
- Cigarette smoking:
- Strongest known risk factor
- Smokers have a 7 to 8× increased risk compared with non-smokers:
- Risk correlates with duration and intensity of exposure
- Cigarette smoking:
- Possible / Associated Risk Factors:
- Older age
- Male gender (historically)
- Prior radiation exposure:
- Weak association:
- Far less than pleomorphic adenoma
- Weak association:
- Chronic inflammatory or immune-related processes (hypothesized, not proven)
- Established:
- Pathology:
- Gross Pathology:
- Well-circumscribed, encapsulated, soft mass
- Frequently cystic, often containing brown, turbid (“motor oil”) fluid
- Histopathology (defining features):
- Biphasic tumor composed of:
- Epithelial component
- Papillary and cystic architecture
- Bilayered oncocytic epithelium – luminal columnar oncocytic cells, basal cuboidal cells
- Lymphoid stroma:
- Dense lymphoid tissue with germinal centers
- Epithelial component
- No true myoepithelial component
- Mitoses and atypia are absent in classic WT
- Biphasic tumor composed of:
- Gross Pathology:
- Molecular Features:
- Unlike pleomorphic adenoma, lacks recurrent driver translocations
- Mitochondrial DNA mutations described (consistent with oncocytic phenotype)
- Increasing evidence suggests WT may represent a tumor-like reactive process rather than a true neoplasm
- Clinical Presentation:
- Painless, slow-growing preauricular or infra-auricular mass
- Often fluctuant due to cystic nature
- Facial nerve dysfunction is exceptional and should raise concern for alternative diagnoses
- Bilateral or synchronous contralateral lesions strongly suggest WT
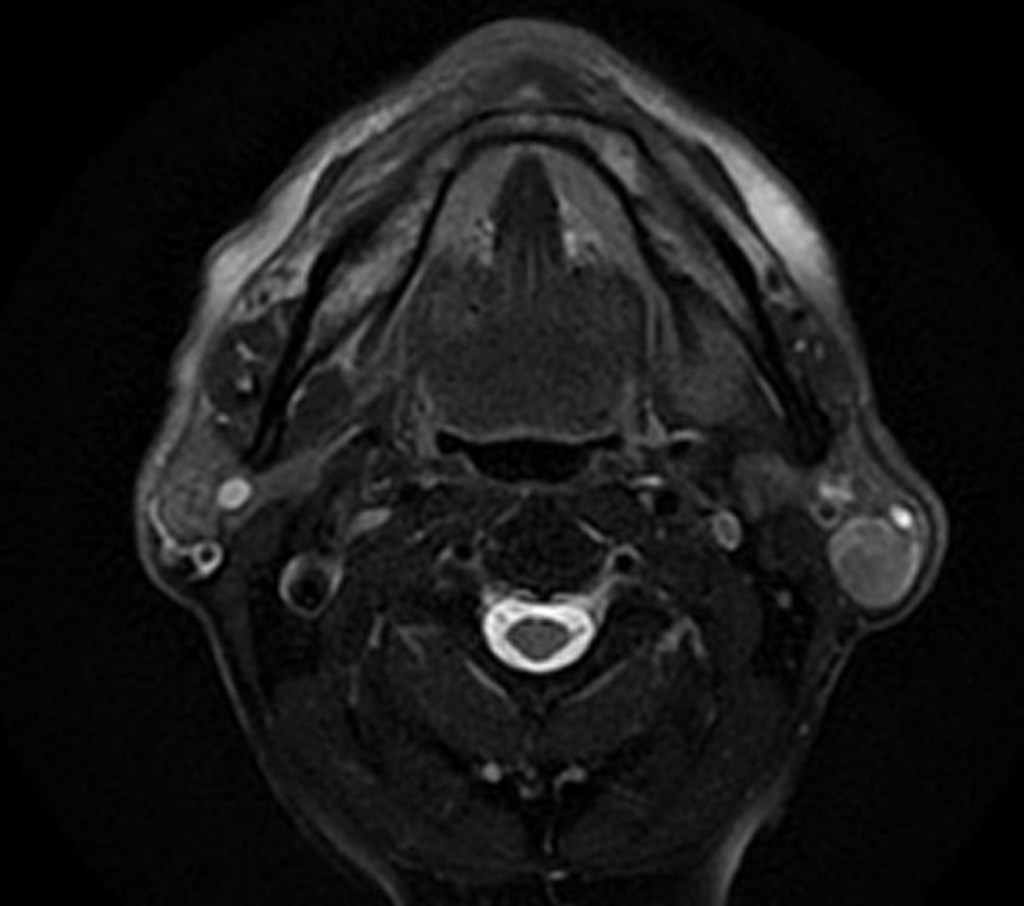
- Imaging Characteristics (supportive, not diagnostic):
- Ultrasound:
- Well-defined, hypoechoic, often cystic with internal septations
- CT:
- Well-circumscribed, cystic or cystic-solid lesion, tail of parotid
- MRI:
- T1: low–intermediate signal
- T2: heterogeneous, often high signal
- Characteristically shows high uptake on Tc-99m pertechnetate scans (classic but rarely used today)
- Ultrasound:
- Diagnosis:
- Fine-needle aspiration (FNA) is usually sufficient:
- Typical findings:
- Oncocytic epithelial cells + lymphoid background
- Diagnostic accuracy is high when classic features are present
- Typical findings:
- Core needle biopsy rarely needed
- Important to correlate with imaging and clinical setting (older smoker, tail of parotid)
- Fine-needle aspiration (FNA) is usually sufficient:
- Management:
- Observation:
- Appropriate in selected patients when:
- Diagnosis is secure:
- Concordant clinical + imaging + FNA
- Asymptomatic or minimally symptomatic
- No cosmetic concern
- No facial nerve dysfunction
- Patient preference supports surveillance
- Diagnosis is secure:
- Rationale:
- Benign behavior
- Very low malignant transformation risk
- Many tumors grow minimally or plateau
- Appropriate in selected patients when:
- Surgical Management:
- Indications:
- Diagnostic uncertainty
- Symptomatic tumor:
- Pain, rapid growth, infection
- Cosmetic deformity
- Patient anxiety or preference
- Very large lesions
- Surgical options:
- Partial superficial parotidectomy
- Extracapsular dissection (ECD) in well-selected cases
- Facial nerve preservation is standard
- Total parotidectomy rarely required
- Neck dissection:
- Not indicated
- Adjuvant therapy:
- None
- Indications:
- Observation:
- Prognosis:
- Excellent
- Recurrence is uncommon and usually reflects:
- Multifocal disease
- Development of a new metachronous WT
- Long-term survival equivalent to general population
- Key Teaching Points for Surgeons:
- Tail of parotid + smoker + cystic mass = think Warthin
- Bilaterality strongly favors WT
- Observation is acceptable and evidence-based in selected patients
- Avoid overtreatment:
- Facial nerve morbidity must be weighed against benign biology
- Key References:
- Barnes L, Eveson JW, Reichart P, Sidransky D. WHO Classification of Tumours of the Head and Neck. IARC Press; 2017 / 2022 (5th ed.).
- Ellis GL, Auclair PL. Tumors of the Salivary Glands. AFIP Atlas of Tumor Pathology.
Seifert G, Donath K. The Warthin tumor: a multifocal disease. Virchows Arch A Pathol Anat Histopathol. 1996. - Eveson JW, Cawson RA. Warthin’s tumour (cystadenolymphoma) of salivary glands: a study of 78 cases. Oral Surg Oral Med Oral Pathol.
- Schwalje AT, Uzelac A, Ryan WR. Growth rate characteristics of Warthin tumors. Otolaryngol Head Neck Surg. 2015.
- Quer M, et al. ESMO–EURACAN Clinical Practice Guidelines for salivary gland cancer. Ann Oncol. 2022.
- Witt RL, et al. Observation of Warthin tumors: a safe alternative to surgery. Otolaryngol Head Neck Surg.