- Significant increase in incidence after mammography screening:
- 17-fold increase from 1970’s to 2004:
- 1 in 1300 mammograms
- Represents 20% of all screen-detected breast neoplasias diagnosed annually
- 17-fold increase from 1970’s to 2004:
- DCIS by it self:
- Is not a risk to life
- DCIS may progress to invasion and compromise survival:
- If left untreated:
- 1 in 6 DCIS patients:
- Progress to invasive breast cancer (IBC):
- 70% estimated to remain indolent
- Progress to invasive breast cancer (IBC):
- 1 in 6 DCIS patients:
- If left untreated:
- At this time we do not have any robust biomarkers:
- That can quantify the risk of progression to IBC or
- Help us separate indolent disease:
- From the potentially dangerous lesions
- Risk of overtreament:
- The increase incidence of DCIS in mammographically detected cases:
- Has not lead to a decrease in the incidence of IBC or reduction of IBC morality
- The increase incidence of DCIS in mammographically detected cases:
- Risk factors for progression / recurrence of DCIS:
- The risk factors for IBC recurrence may be different from the risks factors for DCIS recurrence?
- Risk of IBC recurrence:
- African American race
- Premenopausal status
- Detection by palpation
- Involved margins
- High histologic grade
- High p16 expression
- Risk of IBC or DCIS recurrence:
- DCIS size
- Histology type
- Comedo necrosis
- Grade
- Young age
- Close margins or positive margins
- Risk of IBC recurrence:
- Patients with DICIS that recur with an IBC:
- Some patients with DCIS may develop:
- Progression of there disease
- Some will have a de novo invasive breast cancer
- Some might have a missed invasive cancer?
- Some patients with DCIS may develop:
- Patient that have a DCIS recurrence:
- Might be a true in situ recurrence
- De novo DCIS
- Residual disease?
- Studies are describing observations of events:
- Synchronous IBC
- Subsequent ipsilateral or contralateral DCIS or IBC (often a mixture)
- We have limited data on DCIS progression with paired molecular profiles
- The risk factors for IBC recurrence may be different from the risks factors for DCIS recurrence?
- The most consistent biological feature of DCIS:
- Heterogeneity:
- In clinical presentation
- Morphology
- Protein expression:
- Including receptor status
- Gene expression
- Genetic alterations
- Epigenetic alterations
- The heterogeneity is:
- Between patients – within the lesion – and within cells in a single duct
- Heterogeneity:
- Morphological features that help us predict progression is:
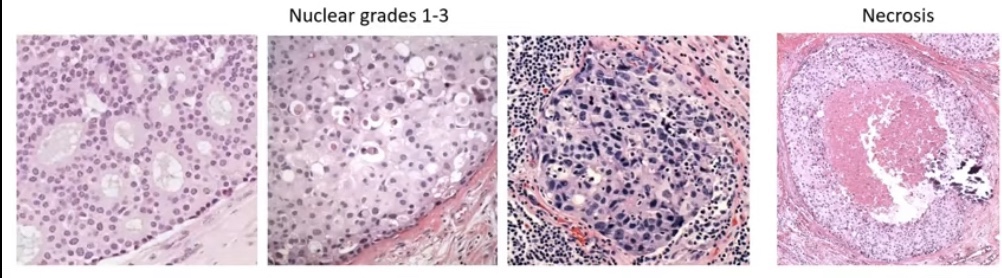
- Histologic grading:
- Combing the nuclear grade 1 to 3 and necrosis into a three tier system (the good, the bad, and the ugly):
- Low / intermediate / high grade
- Grade 1 to 3
- Van Nuys Group 1 to 3
- DIN 1 to 3
- We all know that there is regression towards the mean and substantial interobserver variation
- Combing the nuclear grade 1 to 3 and necrosis into a three tier system (the good, the bad, and the ugly):
- Histologic grading:
- Unclear prognostic value of the 3-tier system:
- We suspect that:
- Low to intermediate grade = low risk of progression
- High grade system = high risk of progression or shorter time to progression
- Maxwell, A.J. Eur.J.Surg.Oncol.,2018, Ryser, MD. J.Natl Cancer Inst., 2019:
- Risk of ipsilateral recurrence (DCIS / IBC) at 10 years:
- High grade 17.6% (95% CI=12.1-25.2%)
- Non high grade 12.2 (95% CI=8.6-17.1%):
- Including grade 2
- There is overlap in the confidence intervals
- Risk of ipsilateral recurrence (DCIS / IBC) at 10 years:
- Low grade DCIS are the lesions that might have:
- Discontinuous growth and skip lesions that might lead to a:
- Greater likelihood of residual disease and recurrence?
- Discontinuous growth and skip lesions that might lead to a:
- Heterogeneity of grade within a lesion
- We suspect that:
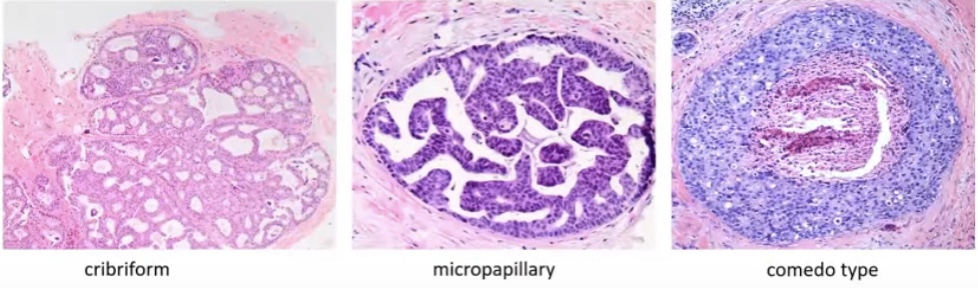
- Histology subtype as a prognostic factor:
- Subtype:
- Cribriform is more often a grade 1 lesion
- Comedo type is more often a grade 3 lesion
- Usually histology subtype correlates with grade but:
- There is often a mixture of growth patterns:
- Compromising the use for prognostication
- There is often a mixture of growth patterns:
- Subtype:
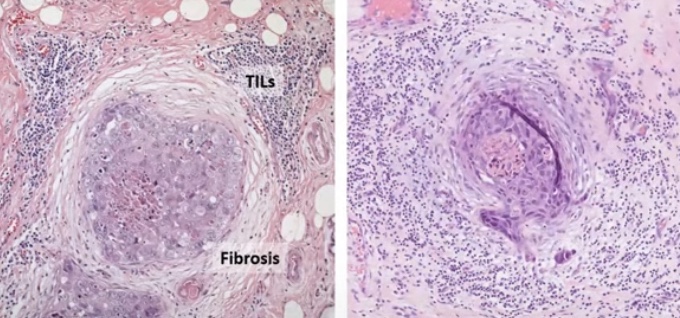
- Tumor micro environment:
- Could potentially be the most important morphologic feature suggestive of progression especially:
- Circumferential periductal fibrosis and associated tumor infiltrating lymphocytes (TIL):
- Indicating host reaction to the tumor cells
- Circumferential periductal fibrosis and associated tumor infiltrating lymphocytes (TIL):
- Tumor micro environment includes:
- Myoepithelial cell layer
- Tumor infiltrating lymphocytes (TIL)
- Adipocytes
- Fibroblasts
- Matrix
- Could potentially be the most important morphologic feature suggestive of progression especially:
- The myoepithelial layer acts as a gatekeeper:
- Has tumor suppressive functions
- The largest gene expression change from normal tissue to DCIS:
- Occurs in the myoepithelial layer
- DCIS associated myoepithelial loss:
- That leads the decrease tumor suppressor functions
- The myoepithelial layer is lost in IBC
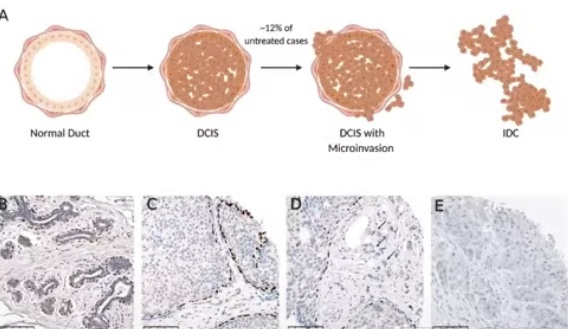
- Disruption of the myoepithelial defense:
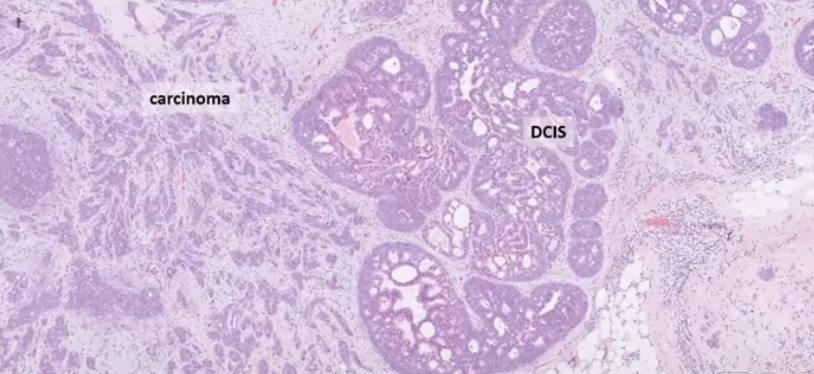
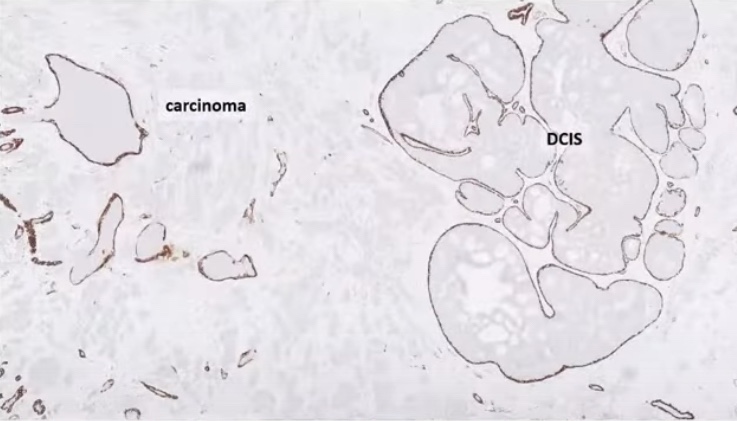
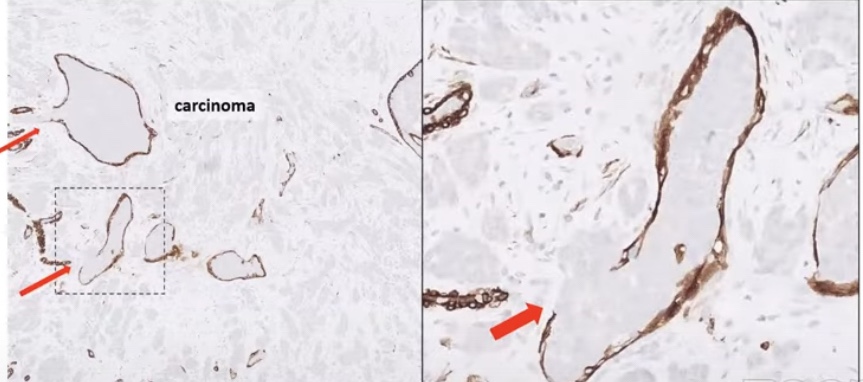
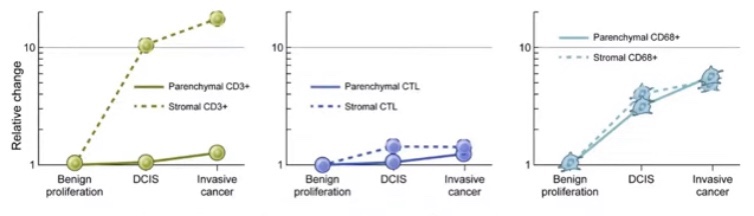
- Conflicting data on prognostic value of TIL:
- Some studies have reported no prognostic value of stromal TIL for subsequent recurrences:
- Does the spatial location of the immune cells matter?
- The TIL in direct contact with the DCIS might be more important that the TIL that are further away
- Does the spatial location of the immune cells matter?
- Other studies have shown a correlation between higher levels of TIL and increased risk of subsequent IBC and a shorter (ipsilateral) recurrence-free survival
- Some studies have reported no prognostic value of stromal TIL for subsequent recurrences: