- Atypical ductal hyperplasia (ADH):
- Is a benign proliferative breast lesion:
- Characterized by filling and distention of ducts by dysplastic monotonous epithelial cells:
- Forming architecturally complex patterns, including:
- Cribriform-like secondary lumens or micropapillary formations
- Forming architecturally complex patterns, including:
- Characterized by filling and distention of ducts by dysplastic monotonous epithelial cells:
- It is found in approximately 10% of benign breast biopsies
- It confers a four-fold increased risk of subsequent breast cancer:
- With a cumulative incidence approaching 30% at 25 years
- Is a benign proliferative breast lesion:
- Definition and Histopathology:
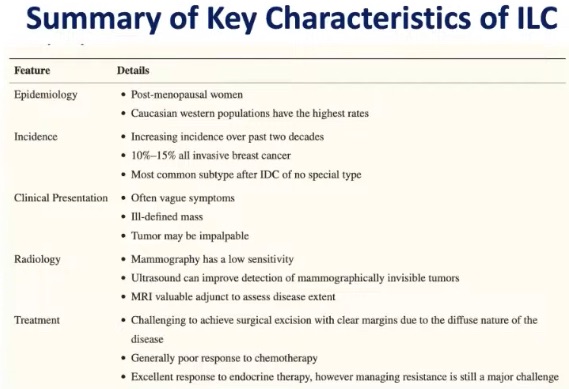
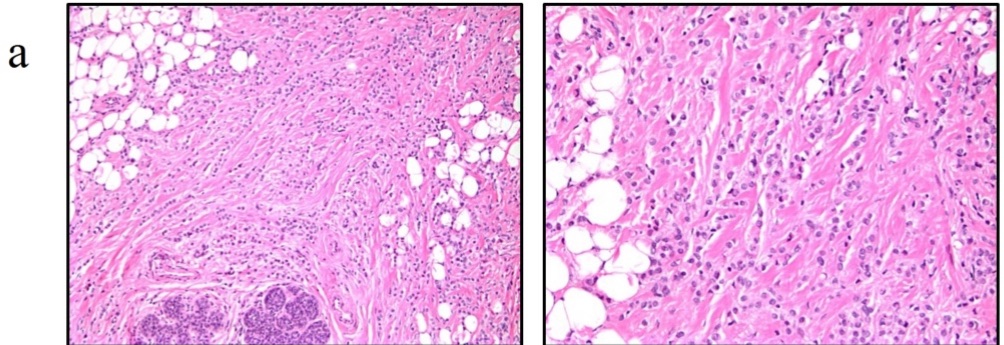
- ADH is defined by cytologic and architectural features:
- Established by Page and colleagues in 1985
- The lesion shows:
- Proliferation of dysplastic, monotonous epithelial cells:
- With architectural complexity and nuclear hyperchromasia
- Proliferation of dysplastic, monotonous epithelial cells:
- The key distinction from ductal carcinoma in situ (DCIS) is quantitative rather than qualitative:
- ADH shares histologic features with low-grade DCIS but is less extensive
- If the lesion meets criteria for DCIS in terms of quality but involves fewer than two ducts or measures less than 2 mm:
- It is classified as ADH
- This places ADH in a transitional zone between benign and malignant disease:
- Making it a premalignant lesion
- ADH is defined by cytologic and architectural features:
- Epidemiology:
- ADH:
- Is found in approximately 10% of core needle biopsy specimens with benign findings
- Both atypical ductal and atypical lobular hyperplasia:
- Occur with equal frequency and confer similar breast cancer risks
- The lesion is typically discovered incidentally on screening mammography:
- In asymptomatic women
- ADH:
- Risk Factors and Modifiers:
- Younger age at diagnosis:
- Is associated with higher subsequent breast cancer risk
- Family history of breast cancer:
- May increase risk, though data are conflicting
- Number of atypical foci significantly impacts risk:
- Women with ≥ 3 foci have a standardized incidence ratio (SIR) of 5.29 compared to 3.11 for a single focus
- Dense breasts:
- Increase risk compared to fatty breasts
- Younger age at diagnosis:
- Imaging Characteristics:
- ADH:
- Has no pathognomonic imaging appearance and typically mimics findings seen in small cancers
- Mammographic Features:
- Clustered microcalcifications:
- Are the most common finding directly correlated with ADH:
- Present in 64% to 82% of cases
- Calcifications of intermediate concern or higher probability of malignancy:
- Are more frequent when ADH is associated with malignancy
- Are the most common finding directly correlated with ADH:
- May also present as masses, asymmetric densities, or architectural distortion
- Direct mammographic-histologic correlation:
- Occurs in approximately 41% of cases
- Clustered microcalcifications:
- Ultrasound Features:
- Most lesions appear as hypoechoic masses (64%)
- Irregular shape (51%) and microlobulated margins (49%)
- No specific posterior acoustic features (53%)
- Parallel orientation (57%)
- Presence of calcifications on ultrasound is significantly associated with upgrade to malignancy
- ADH lesions are typically assigned BI-RADS category 4 (suspicious abnormality):
- Warranting tissue sampling by core needle biopsy
- ADH:
- Management:
- Surgical Excision:
- Surgical excision remains the standard of care for ADH diagnosed on core needle biopsy:
- Due to upgrade rates of 15% to 30% to DCIS or invasive cancer
- A 2020 meta-analysis of 6,458 lesions:
- Found a 29% upgrade rate for surgically excised ADH
- The Society of Surgical Oncology recommends routine excision:
- Noting an upgrade rate of at least 20%
- Surgical excision remains the standard of care for ADH diagnosed on core needle biopsy:
- Emerging Evidence for Selective Observation:
- Recent literature suggests that select low-risk ADH lesions may be candidates for observation rather than routine excision:
- Lesions that appear completely removed at biopsy
- Limited foci:
- Fewer than 2 to 3 foci
- No necrosis or significant atypia on pathology
- Small groups of mammographic calcifications
- No enhancement on MRI
- No underlying risk factors:
- No history of breast cancer
- No genetic mutation
- No concurrent high-risk lesions
- Recent literature suggests that select low-risk ADH lesions may be candidates for observation rather than routine excision:
- A 2022 study found that selected women with ADH who met predetermined low-risk criteria and were managed nonoperatively:
- Had a 1.2% index site cancer rate at median 5.2-year follow-up:
- Comparable to the 1.5% rate in those who underwent surgery
- Had a 1.2% index site cancer rate at median 5.2-year follow-up:
- A 2025 study applying COMET trial criteria:
- Found only a 3.43% upgrade to invasive disease in low-risk patients
- Surgical Excision:
- Post-Diagnosis Management:
- For women with confirmed ADH on excisional biopsy:
- Enhanced surveillance:
- Annual mammography plus breast MRI screening
- Risk-reducing medications:
- Endocrine therapy (tamoxifen or aromatase inhibitors) is strongly recommended by NCCN guidelines:
- With an 86% risk reduction for women with atypical hyperplasia
- Lifestyle modifications:
- Counseling on healthy lifestyle factors
- Enhanced surveillance:
- Prognosis:
- ADH confers a relative risk of approximately 4 for future breast cancer compared to women without the diagnosis
- The absolute risk is substantial and sustained over time:
- Cumulative Breast Cancer Incidence:
- 5 years: 6.6% (95% CI 4.4-9.7%)
- 10 years: 13.9% (95% CI 7.8-23.6%)
- 25 years: 30% (either DCIS or invasive cancer)
- The 10-year cumulative incidence is approximately 14.6%:
- Representing about 1% per year
- Risk increases with the number of atypical foci present:
- Women with ≥ 3 foci have nearly double the risk of those with a single focus
- Cumulative Breast Cancer Incidence:
- Important Prognostic Considerations:
- Risk affects both the ipsilateral and contralateral breast:
- Though ipsilateral risk may be slightly higher
- The risk does not plateau but continues to increase linearly over decades
- Approximately half of subsequent breast cancers:
- Occur within the first 5 years after ADH diagnosis
- Both DCIS and invasive cancer contribute to subsequent events
- The NCCN Breast Cancer Risk Reduction guidelines:
- Classify women with atypical hyperplasia as high-risk and recommend risk-reducing endocrine therapy for those with life expectancy ≥ 10 years, given the substantial and sustained elevation in breast cancer risk
- Risk affects both the ipsilateral and contralateral breast:
- For women with confirmed ADH on excisional biopsy:
- References:
- Atypical Hyperplasia of the Breast — Risk Assessment and Management Options. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. The New England Journal of Medicine. 2015;372(1):78-89. doi:10.1056/NEJMsr1407164.
- Updates on Management of Atypical Hyperplasia of the Breast. Klassen CL, Fraker JL, Pruthi S. Mayo Clinic Proceedings. 2025;100(6):1051-1057. doi:10.1016/j.mayocp.2025.01.029.
- Subsequent Breast Cancer Risk Following Diagnosis of Atypical Ductal Hyperplasia on Needle Biopsy. Menes TS, Kerlikowske K, Lange J, et al. JAMA Oncology. 2017;3(1):36-41. doi:10.1001/jamaoncol.2016.3022.
- Benign Breast Disease and Breast Cancer Risk in the Percutaneous Biopsy Era. Sherman ME, Vierkant RA, Winham SJ, et al. JAMA Surgery. 2024;159(2):193-201. doi:10.1001/jamasurg.2023.6382.
- Atypical Hyperplasia of the Breast: Mammographic Appearance and Histologic Correlation. Helvie MA, Hessler C, Frank TS, Ikeda DM. Radiology. 1991;179(3):759-64. doi:10.1148/radiology.179.3.2027988.
- Imaging Characteristics of and Multidisciplinary Management Considerations for Atypical Ductal Hyperplasia and Flat Epithelial Atypia: Review of Current Literature. Harper LK, Carnahan MB, Bhatt AA, et al. Radiographics : A Review Publication of the Radiological Society of North America, Inc. 2023;43(10):e230016. doi:10.1148/rg.230016.
- Atypical Ductal Hyperplasia Diagnosed at Sonographically Guided Core Needle Biopsy: Frequency, Final Surgical Outcome, and Factors Associated With Underestimation. Mesurolle B, Perez JC, Azzumea F, et al. AJR. American Journal of Roentgenology. 2014;202(6):1389-94. doi:10.2214/AJR.13.10864.
- Mucocele-Like Tumors of the Breast as Cystic Lesions: Sonographic-Pathologic Correlation. Kim SM, Kim HH, Kang DK, et al. AJR. American Journal of Roentgenology. 2011;196(6):1424-30. doi:10.2214/AJR.10.5028.
- Diagnosis of Columnar Cell Lesions and Atypical Ductal Hyperplasia by Ultrasound-Guided Core Biopsy: Findings Associated With Underestimation of Breast Carcinoma. Ahn HS, Jang M, Kim SM, et al. Ultrasound in Medicine & Biology. 2016;42(7):1457-63. doi:10.1016/j.ultrasmedbio.2016.02.009.
- Society of surgical oncology medical student & trainee primer for breast surgical oncology. Marissa K. Boyle, Julia M. Selfridge, Rachel E. Sargent, et al.
Upgrade Rate of Percutaneously Diagnosed Pure Atypical Ductal Hyperplasia: Systematic Review and Meta-Analysis of 6458 Lesions. Schiaffino S, Calabrese M, Melani EF, et al. Radiology. 2020;294(1):76-86. doi:10.1148/radiol.2019190748. - Risk of Breast Cancer in Selected Women With Atypical Ductal Hyperplasia Who Do Not Undergo Surgical Excision. Kilgore LJ, Yi M, Bevers T, et al. Annals of Surgery. 2022;276(6):e932-e936. doi:10.1097/SLA.0000000000004849.
- Implications of the COMET Trial for the Management of Atypical Ductal Hyperplasia. Zaveri S, Sun SX, Bevers TB, Albarracin CT, Bedrosian I. Annals of Surgical Oncology. 2025;:10.1245/s10434-025-18236-2. doi:10.1245/s10434-025-18236-2.
- Atypical Hyperplasia of the Breast: Clinical Cases and Management Strategies. Vegunta S, Mussallem DM, Kaur AS, Pruthi S, Klassen CL. Cleveland Clinic Journal of Medicine. 2023;90(7):423-431. doi:10.3949/ccjm.90a.22098.
Breast Cancer Risk Reduction. National Comprehensive Cancer Network. Updated 2025-08-29. - Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. Visvanathan K, Fabian CJ, Bantug E, et al. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2019;37(33):3152-3165. doi:10.1200/JCO.19.01472.
Practice Bulletin Number 179: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstetrics and Gynecology. 2017;130(1):e1-e16. doi:10.1097/AOG.0000000000002158. - Atypical Ductal or Lobular Hyperplasia, Lobular Carcinoma in-Situ, Flat Epithelial Atypia, and Future Risk of Developing Breast Cancer: Systematic Review and Meta-Analysis. Baker J, Noguchi N, Marinovich ML, et al. Breast (Edinburgh, Scotland). 2024;78:103807. doi:10.1016/j.breast.2024.103807.
- Trajectory of Subsequent Breast Cancer Diagnoses in a Diverse Patient Cohort With Breast Atypia. Limberg JN, Thomas SM, Dalton JC, et al. Annals of Surgical Oncology. 2024;31(11):7550-7558. doi:10.1245/s10434-024-15788-7.