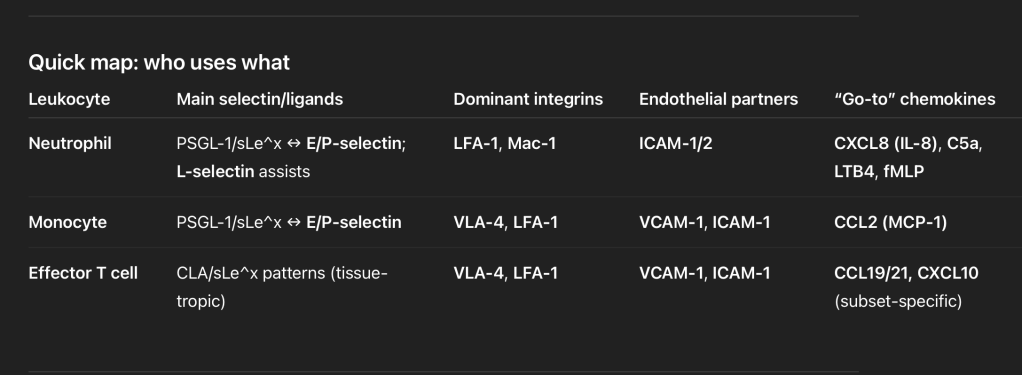
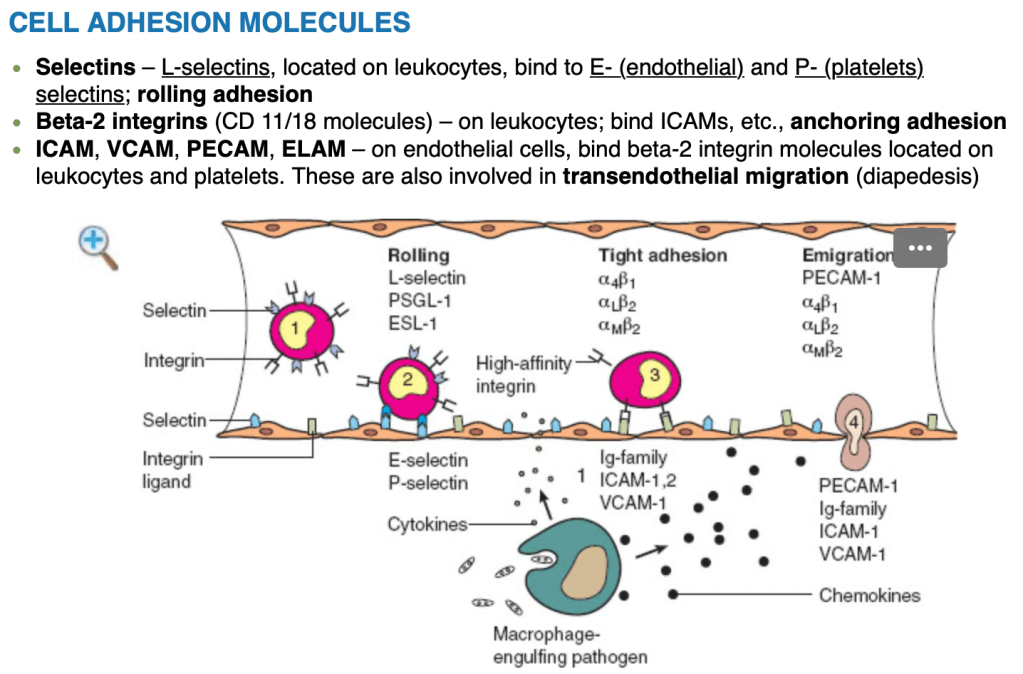
- Atypical ductal hyperplasia (ADH):
- Proliferative breast lesions:
- Such as ADH confer a substantial increase in breast cancer risk of:
- 4 to 5-fold when compared to the general population (RR 3.7-5.3)
- Such as ADH confer a substantial increase in breast cancer risk of:
- Proliferative breast lesions:
- In post-menopausal women a higher BMI and / or peri-menopausal weight gain:
- Is associated with a higher risk of breast cancer
- In the Nurses’ Health Study:
- Women who gained 10 kg or more after menopause had a greater risk of developing breast cancer compared to those who maintained their weight (RR 1.18, 95% CI 1.03-1.35)
- After an average of 10 years of follow-up in the Women’s Health Study:
- Higher daily alcohol consumption was associated with an increase in invasive breast cancer risk (RR 1.43, 95% CI 1.02-2.02 for ≥ 30g alcohol (2 to 3 drinks vs. none)
- Based on epidemiological data from 52 studies, risk ratios for breast cancer increase with the number of affected first-degree relatives:
- 1.8 (99% CI 1.7-1.9), 2.9 (2.4-3.6), and 3.9 (2.0-7.5) respectively for one, two, and three or more affected relatives vs. none
- There is no definitive evidence that caffeine intake is associated with breast cancer risk
- References
- Smart CE, Furnival CM, Lakhani SR. Chapter 17: High-Risk Lesions: ALH/LCIS/ADH. In: Kuerer HM, ed. Kuerer’s Breast Surgical Oncology. New York, NY: McGraw-Hill; 2010.
- Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201.
- Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol. 2007;165(6):667-676.
- Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389-1399.
- Ganmaa D, Willett WC, Li TY, et al. Coffee, tea, caffeine and risk of breast cancer: a 22-year follow-up. Int J Cancer. 2008;122(9):2071-2076.