Choledochal Cysts – Types and Management
Choledochal cysts are congenital cystic dilatations of the biliary tree. They are associated with an abnormal pancreaticobiliary junction and carry a significant lifetime risk of malignancy (especially cholangiocarcinoma).
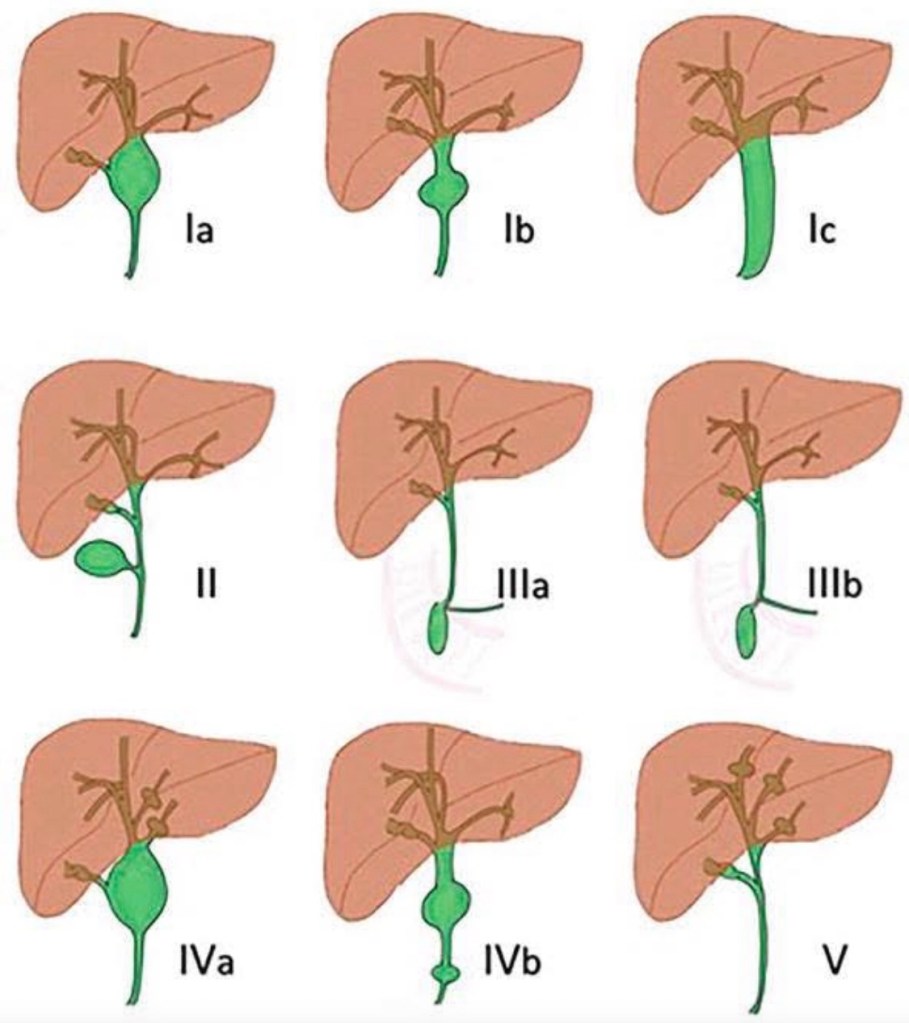
Classification (Todani Classification)
The most widely used system is the Todani classification, which divides choledochal cysts into five main types:
Type I – Extrahepatic bile duct dilatation (most common, 50–80%)
• Ia – Diffuse cystic dilatation of CBD
• Ib – Focal segmental dilatation
• Ic – Fusiform dilatation of CBD
Management:
→ Complete excision of extrahepatic bile duct + Roux-en-Y hepaticojejunostomy
Type II – True diverticulum of CBD
• Saccular outpouching from extrahepatic bile duct
Management:
→ Diverticulectomy ± primary closure of CBD
Type III – Choledochocele
• Intraduodenal dilatation of distal CBD (within ampulla)
Management:
→ Endoscopic sphincterotomy (often sufficient)
→ Surgical excision if large/symptomatic
Type IV – Multiple cysts
• IVa – Both intrahepatic and extrahepatic involvement
• IVb – Multiple extrahepatic cysts only
Management:
→ Excision of extrahepatic bile duct + Roux-en-Y hepaticojejunostomy
→ Liver resection if localized intrahepatic disease
→ Liver transplant if diffuse severe intrahepatic disease
Type V – Caroli Disease
• Multiple intrahepatic cystic dilatations only
Associated with congenital hepatic fibrosis.
Management:
→ Segmental liver resection (localized)
→ Liver transplantation (diffuse disease)
Clinical Presentation
• Children: classic triad (rarely complete)
• Abdominal pain
• Jaundice
• Palpable mass
• Adults:
• Recurrent cholangitis
• Pancreatitis
• Biliary colic
• Incidental finding
Investigations
• Ultrasound – initial test
• MRCP – investigation of choice
• CT if malignancy suspected
• LFTs
ERCP mainly therapeutic (type III).
Complications
• Cholangitis
• Pancreatitis
• Stones
• Strictures
• Rupture (rare)
• Cholangiocarcinoma (10–30% lifetime risk if untreated)
Principles of Management (Important for Practice)
- Complete cyst excision whenever possible
- Avoid drainage procedures (obsolete due to cancer risk)
- Long-term follow-up due to residual malignancy risk
- Early surgery in children once diagnosed
Surgical Standard Operation
Cyst excision + Roux-en-Y hepaticojejunostomy
→ Gold standard for Type I and IV