- Paper:
- “Surgical Management of Invasive Differentiated Thyroid Cancer: An Evidence-Based Review”:
- Published in: Thyroid, 2016; Vol 26(9): 1156–1166
[DOI: 10.1089/thy.2015.0567]
- Published in: Thyroid, 2016; Vol 26(9): 1156–1166
- “Surgical Management of Invasive Differentiated Thyroid Cancer: An Evidence-Based Review”:
- Objective:
- To provide an evidence-based review on the frequency, clinical implications, and management strategies of invasive differentiated thyroid cancer (DTC) involving adjacent structures of the neck.
- Key Findings:
- Frequency of Invasion (based on pooled data and institutional experience):
- Recurrent Laryngeal Nerve (RLN): ~ 47% of locally advanced cases
- Strap Muscles: ~ 40%
- Trachea: ~ 21%
- Esophagus: ~ 12%
- Larynx: ~ 3%
- Carotid Artery: ~ 2%
- RLN and strap muscle invasion were:
- The most common sites of local extension.
- Surgical Management Recommendations:
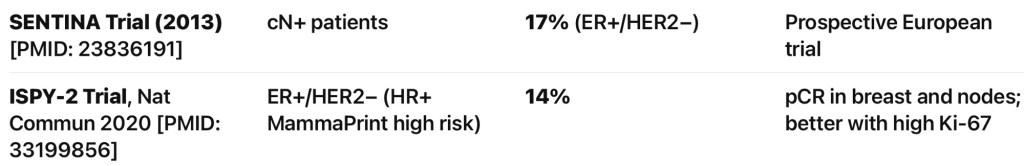
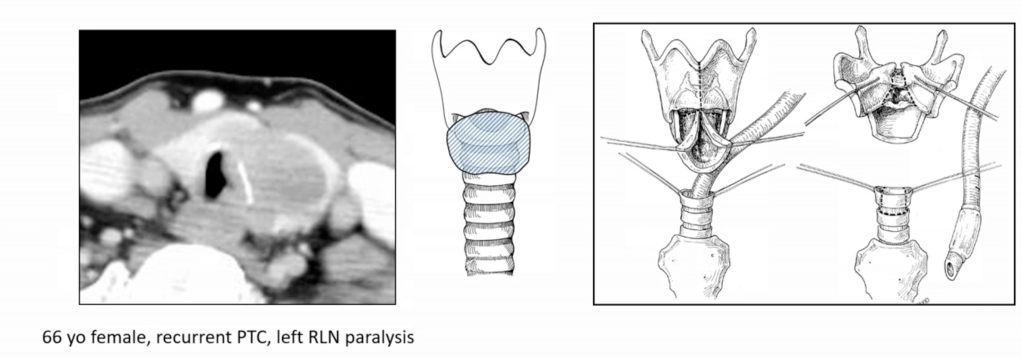
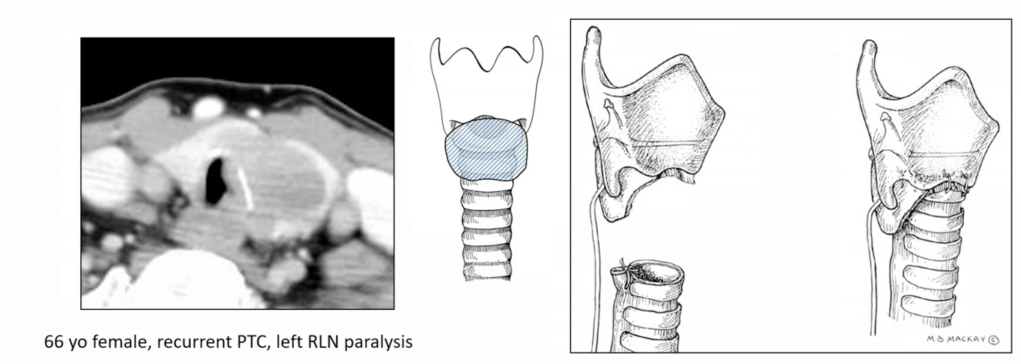
- Recurrent Laryngeal Nerve (RLN):
- If functional and partially encased:
- Consider nerve preservation via shaving
- If non-functional or fully invaded:
- Resection is advised with or without reinnervation techniques
- Postoperative vocal cord assessment is mandatory
- American Head and Neck Society (AHNS) Consensus Statement:
- Statement 2-A:
- RLN encased, ipsilateral vocal cord (VC) paresis / paralysis:
- Resection is indicated (consensus)
- RLN encased, ipsilateral vocal cord (VC) paresis / paralysis:
- Statement 2-B:
- RLN encased, ipsilateral bilateral normal VC function:
- Tumor may be shaved off to spare the RLN, as long as all gross disease is removed (consensus)
- RLN encased, ipsilateral bilateral normal VC function:
- Statement 2-C:
- RLN encased, contralateral VC paretic / paralyzed:
- Tumor may be shaved off so that the RLN is spared (consensus)
- RLN encased, contralateral VC paretic / paralyzed:
- Reference:
- Shindo ML et al. Management of invasive well-differentiated thyroid cancer: An American Head and Neck Society Consensus Statement: AHNS Consensus Statement. Head Neck 2014 36: 1379-1390.
- Statement 2-A:
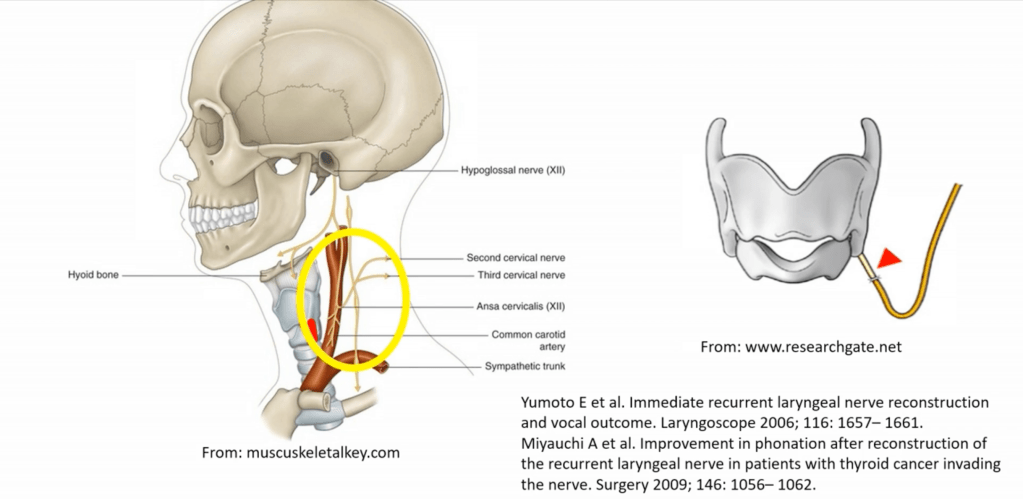
- If the nerve is sacrified RLN reconstruction is advisable or thyroplasty or cord injection.
- If functional and partially encased:
- Recurrent Laryngeal Nerve (RLN):
- Frequency of Invasion (based on pooled data and institutional experience):
- Surgical Management Recommendations:
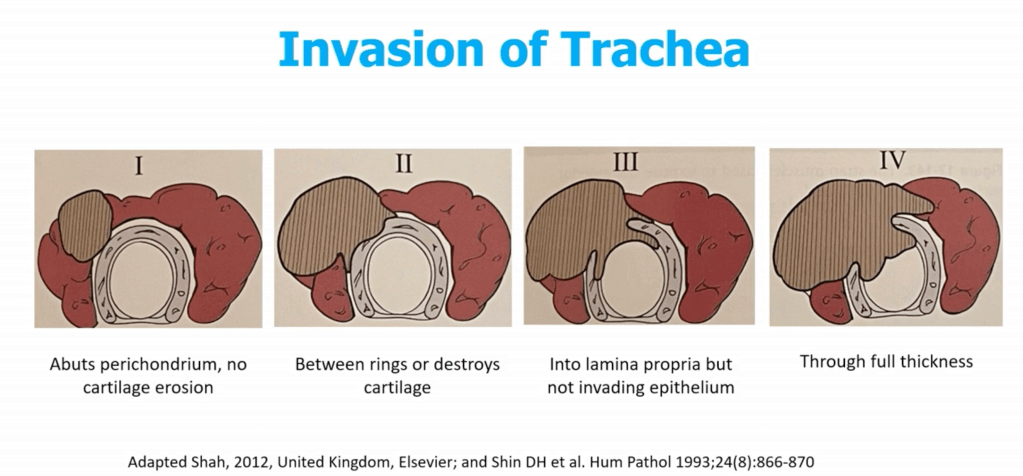
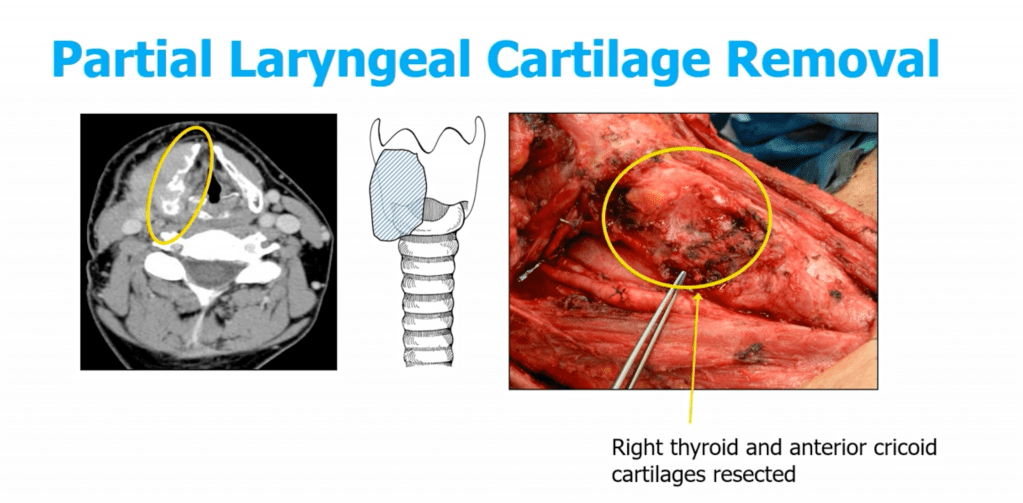
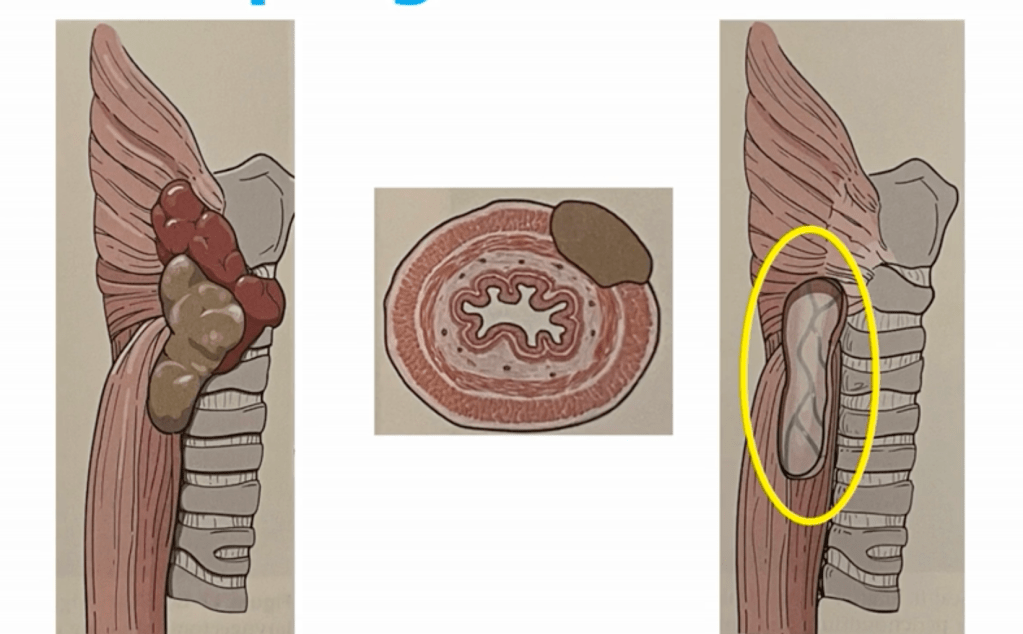
- Trachea:
- Shaving of superficial invasion is acceptable
- Full-thickness invasion may require window or segmental resection (e.g., tracheal reconstruction):
- Multi-disciplinary planning often needed
- Main methods of management:
- Shave:
- Used when tumor invades perichondrium or cartilage only:
- Tangential excision with minimal invasion leaving mucosa intact
- Preserves tracheal framework
- Disadvantages:
- Confirming negative margins intraoperatively
- Lack of continuous plane underneath the external perichondrium
- Tumor spread into the tracheal lumen via lymphatics that communicate in the intercartilaginous space
- Local control:
- Around 95% if tumor does not penetrate beyond the perichondrium
- Window Resection:
- Limited by the length and circumference of the trachea to mantain stability:
- Need to resect < 1/3 of the circumference
- Partial resection of < 3 rings
- Often for McCaffrey Stage II to III
- Primary closure rarely possible
- Needs to be reconstructed with muscle flap or patch graft
- Limited by the length and circumference of the trachea to mantain stability:
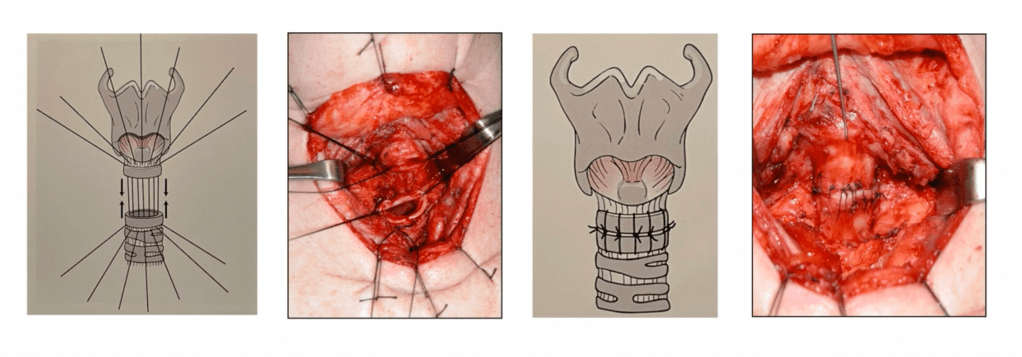
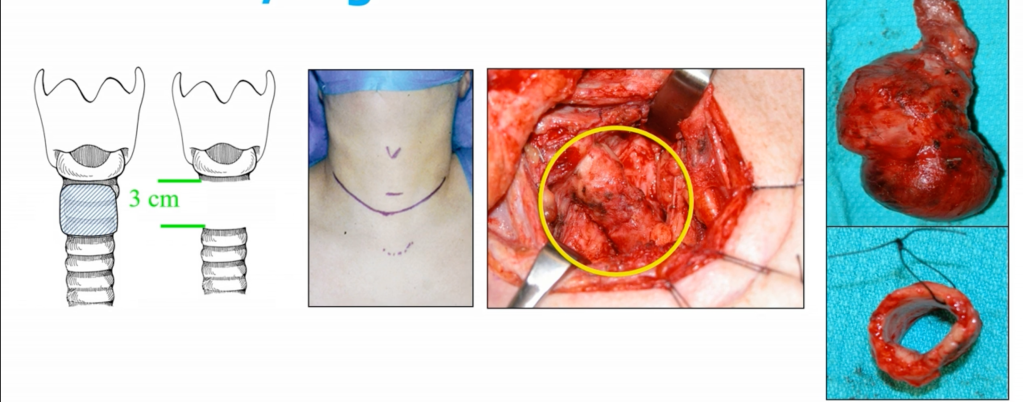
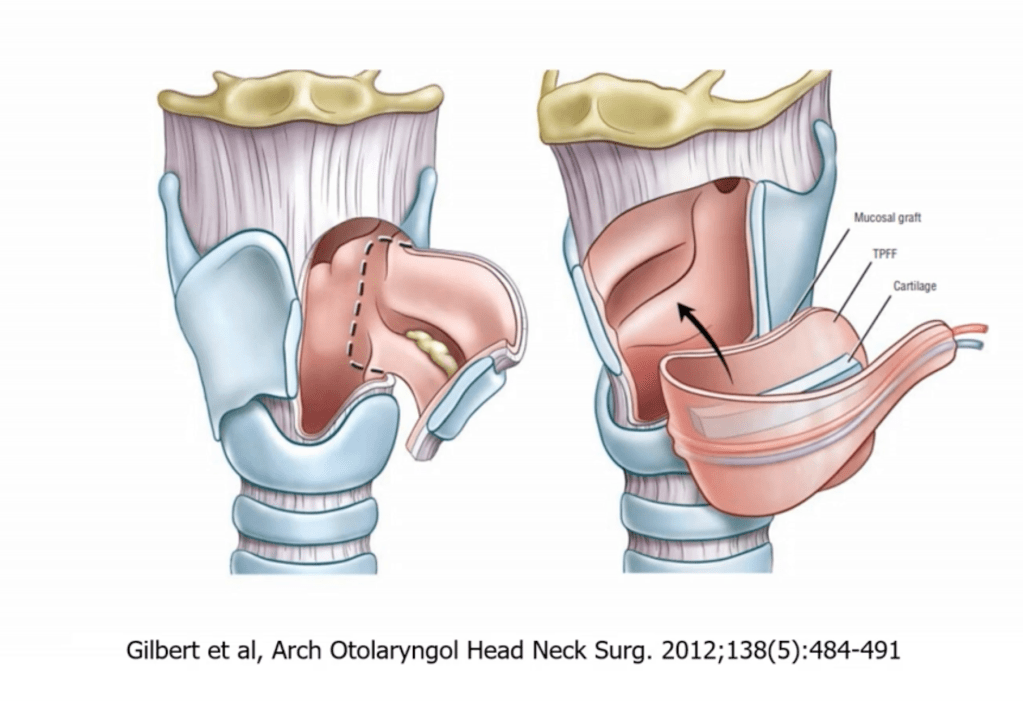
- Sleeve / Segmental Resection:
- En bloc removal of ≥ 2 tracheal rings:
- Up to 5 cm to 6 cm or 5 to 7 rings
- End-to-end primary anastomosis under neck flexion
- May include cricotracheal or laryngotracheal resection if involvement is proximal
- Technical Considerations:
- Maximum safe length for tension-free anastomosis: ~4.5 to 6 cm (5 to 7 rings)
- Requires preoperative anesthesia planning for airway control
- Neck flexion with chin-to-chest sutures post-op
- Close monitoring for anastomotic dehiscence, tracheomalacia, or RLN injury
- Outcomes:
- 5-year disease-specific survival:
- ~ 60% to 75% after R0 sleeve resection
- Morbidity:
- Risk of vocal cord paralysis, anastomotic leak, or stenosis
- Local control better with segmental vs shave resection in deeply invasive tumors
- 5-year disease-specific survival:
- En bloc removal of ≥ 2 tracheal rings:
- Cricotracheal Resection
- Used when tumor invades perichondrium or cartilage only:
- Shave:
- Esophagus:
- Esophageal invasion occurs in approximately 5% to 15% of patients with locally advanced differentiated thyroid cancer (DTC) or poorly differentiated thyroid cancer:
- Particularly with posterior capsular extension from the thyroid gland:
- It is most often associated with invasion of the cervical esophagus and less frequently with thoracic extension
- Particularly with posterior capsular extension from the thyroid gland:
- Assessment and Staging:
- Preoperative Workup:
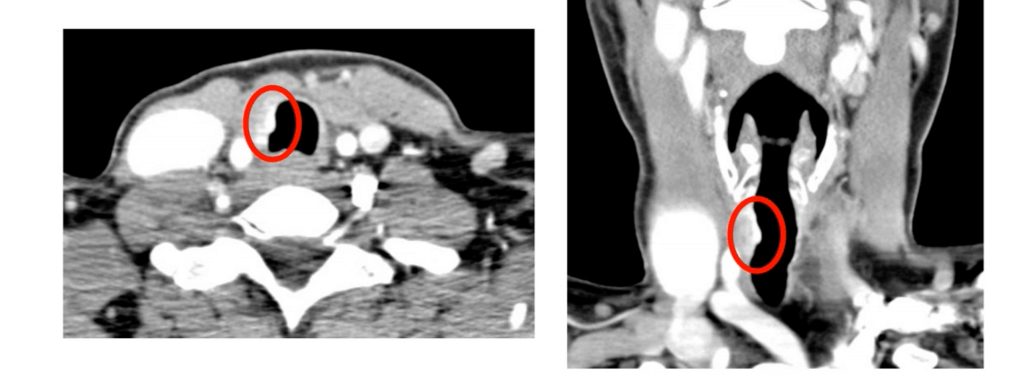
- CT scan with contrast:
- Assess loss of fat plane and wall thickening
- Endoscopic ultrasound (EUS) or esophagoscopy:
- Assess mucosal involvement
- Barium swallow:
- Functional and structural assessment
- Flexible laryngoscopy:
- Assess vocal cord function
- CT scan with contrast:
- McCaffrey Staging System (modified for posterior invasion):
- Stage I to II:
- Abutment or superficial muscular invasion
- Stage III:
- Transmural involvement with mucosal breach
- Stage IV:
- Extensive circumferential or thoracic invasion
- Stage I to II:
- Preoperative Workup:
- Surgical Management:
- Shave Excision:
- For superficial invasion of muscularis layer only:
- Avoids full-thickness resection
- Low morbidity, but risk of residual disease if not adequately evaluated
- For superficial invasion of muscularis layer only:
- Partial Thickness Resection:
- Involves resection of outer muscular layer with cautery or cold dissection
- Often with sternohyoid or SCM flap reinforcement
- Full-Thickness Resection (Segmental Esophagectomy):
- Reserved for mucosal or transmural involvement
- Requires primary closure or flap reconstruction:
- For example radial forearm, pectoralis major, or free jejunal flap
- May require temporary nasogastric / PEG feeding or tracheostomy
- Cervical Esophagectomy with Reconstruction:
- Rare; indicated in extensive disease
- High morbidity, reserved for selected cases with curative intent
- Shave Excision:
- Postoperative Considerations:
- Leak test (methylene blue or contrast swallow) on POD 5 to 7 if full-thickness resection
- Monitor for:
- Dysphagia
- Fistula
- Stricture formation
- Consider gastrostomy or jejunostomy in high-risk cases
- Adjuvant Therapy:
- Radioactive Iodine (RAI):
- If iodine-avid disease and residual / recurrent disease
- External Beam Radiation (EBRT)::
- Gross residual disease
- Positive margins
- Non-RAI-avid disease
- Radioactive Iodine (RAI):
- Prognosis:
- Complete resection (R0) improves local control and survival
- Positive margins or incomplete resection associated with:
- Higher recurrence rates
- Lower disease-specific survival
- Five-year survival can still exceed 60% to 70% with aggressive, multidisciplinary management
- Esophageal invasion occurs in approximately 5% to 15% of patients with locally advanced differentiated thyroid cancer (DTC) or poorly differentiated thyroid cancer:
- Key References:
- McCaffrey TV. Surgical management of invasion into the aerodigestive tract by well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 1999;125(4):401–405.
- Shaha AR. Airway and esophageal involvement in thyroid cancer. World J Surg. 2007;31(5):904–911.
- Nixon IJ et al. Locally advanced thyroid cancer: Surgical management. Thyroid. 2016;26(9):1156–1166.
- Gaissert HA et al. Surgical treatment of invasive thyroid cancer. Ann Thorac Surg. 2007;83(6):1950–1955.
- Haugen BR et al. ATA Guidelines. Thyroid. 2016;26(1):1–133.
- Kim JW et al. Optimal surgical approach to locally invasive DTC. J Surg Oncol. 2017;116(2):229–234.
- McCaffrey TV. Surgical management of laryngotracheal invasion by well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 1999;125(4):401–405.
- Gaissert HA et al. Segmental tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg. 2007;83(6):1950–1955.
- Kim JW et al. Optimal surgical extent for locally invasive thyroid cancer. J Surg Oncol. 2017;116(2):229–234.
- Shaha AR. Airway management in thyroid cancer. World J Surg. 2007;31(5): 903–908.
- Trachea:
- Strap Muscles:
- Often resected without morbidity
- Invasion here does not necessarily confer worse prognosis
- The strap muscles (sternohyoid, sternothyroid, omohyoid, thyrohyoid):
- Lie anterior and lateral to the thyroid gland and are often the first structures invaded in locally advanced disease
- Seen in up to 40% to 50% of cases with extrathyroidal extension (ETE):
- Classified by AJCC 8th edition as:
- Minimal ETE:
- Invasion into perithyroidal soft tissue:
- Not included in T staging
- Invasion into perithyroidal soft tissue:
- Gross ETE to strap muscles:
- T3b disease
- Minimal ETE:
- According to Nixon IJ et al., strap muscle is the most frequently invaded adjacent structure in locally advanced DTC
- Classified by AJCC 8th edition as:
- Diagnosis:
- Clinical and Imaging Features:
- May present as firm fixation of the gland to strap muscles
- On ultrasound CT:
- Loss of fat plane
- Muscle effacement
- Intraoperative findings often determine true invasion
- Clinical and Imaging Features:
- Surgical Management:
- Recommended Approach:
- En bloc resection of involved strap muscles with the thyroid gland
- Usually limited to sternohyoid and sternothyroid
- No need for reconstruction unless deep muscle loss impairs swallowing or airway support
- Not Recommended:
- Piecemeal shaving or curettage:
- May lead to positive margins
- Avoid unnecessarily wide resections if invasion is not gross
- Piecemeal shaving or curettage:
- Pathologic confirmation of muscle invasion is essential for staging (T3b)
- Recommended Approach:
- Oncologic Impact:
- Survival & Recurrence:
- Strap muscle invasion alone does not significantly affect disease-specific survival
- Prognosis more dependent on:
- Nodal status
- Margin status
- Multifocality or vascular invasion
- Kim et al., J Surg Oncol 2017 – strap muscle invasion was not an independent predictor of recurrence or mortality
- Survival & Recurrence:
- Adjuvant Therapy:
- RAI therapy based on full risk stratification (not just muscle invasion)
- No EBRT indicated for strap-only invasion with negative margins
- ATA 2015 Guidelines:
- Strap invasion alone may not upstage to high-risk unless other features are present
- Often resected without morbidity
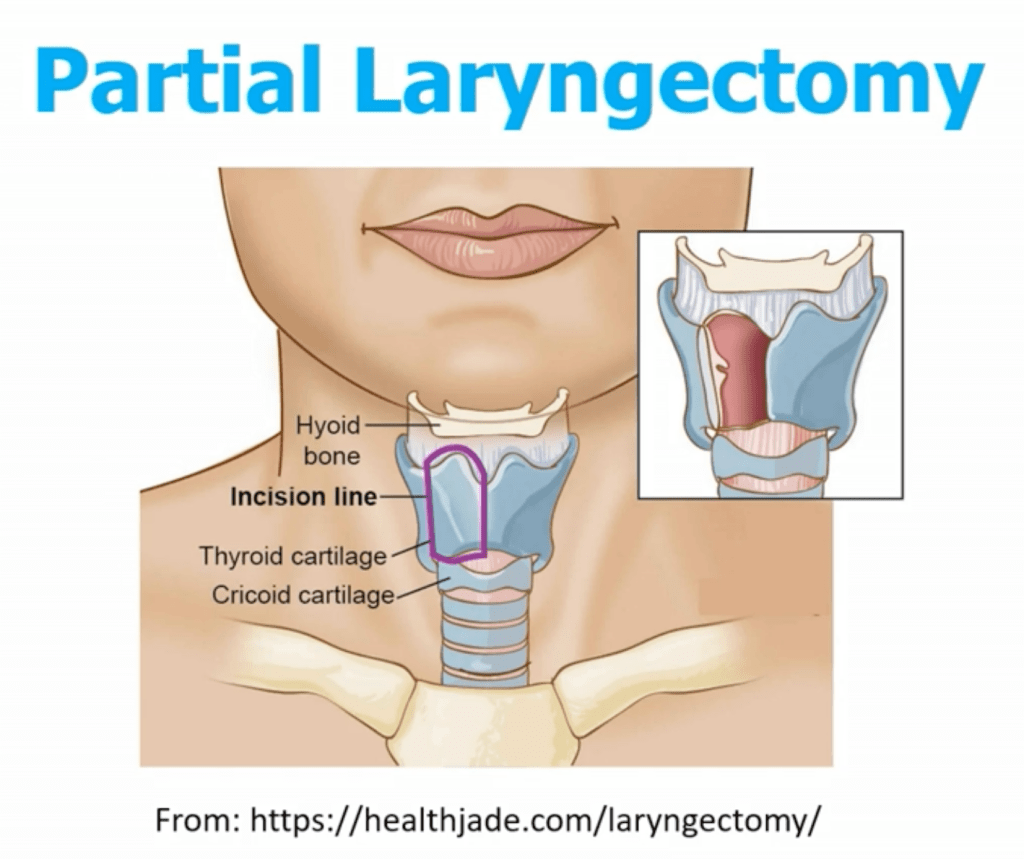
- Larynx and Carotid Artery:
- Invasion is rare but serious
- Laryngectomy or carotid resection is only considered in select patients with curative intent
- Prognosis and Outcomes:
- Gross extrathyroidal extension (T4 disease) is associated with worse disease-specific survival
- However, microscopic invasion alone does not significantly impact survival
- Complete surgical resection remains the most important prognostic factor
- Conclusions:
- Adjacent structure invasion is relatively common in advanced DTC, especially involving the RLN and strap muscles
- Tailored surgical approaches balancing oncologic control and functional preservation are critical
- Multidisciplinary care and evidence-guided surgical decision-making optimize outcomes.

- Conclusion:
- Surgery is the KEY component for survival in patients with poorly differentiated / invasive thyroid cancer
- Not common:
- Consider centers of excellence / high volume