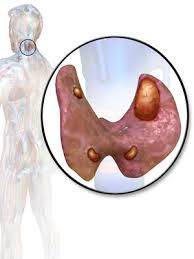
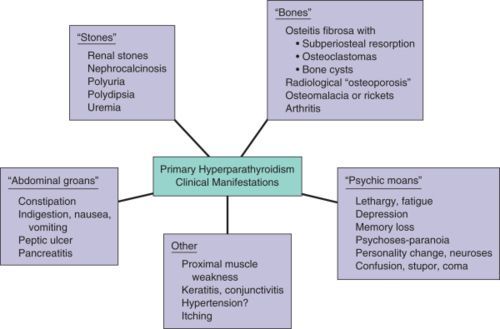
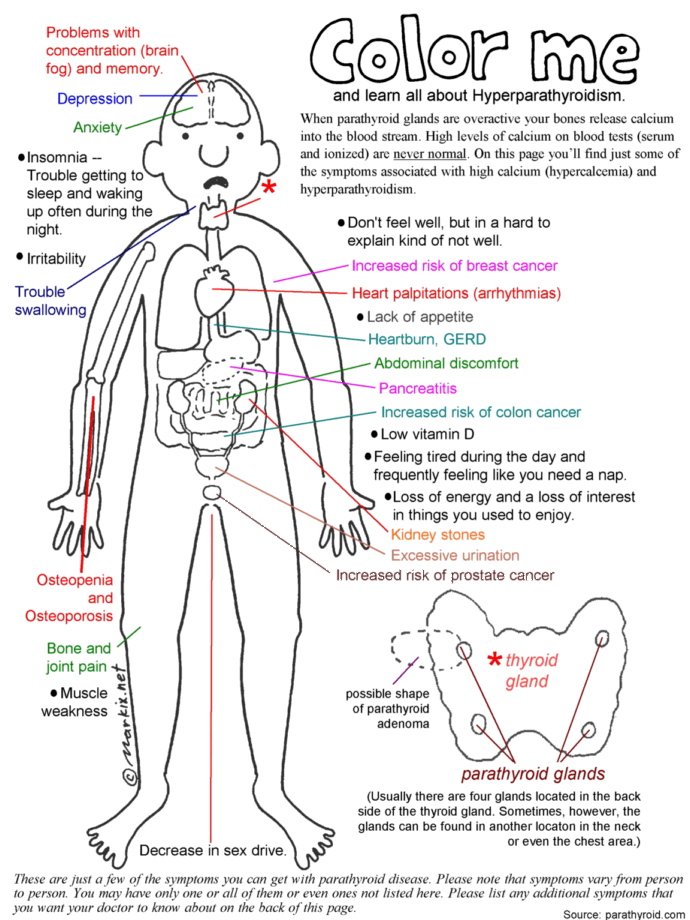
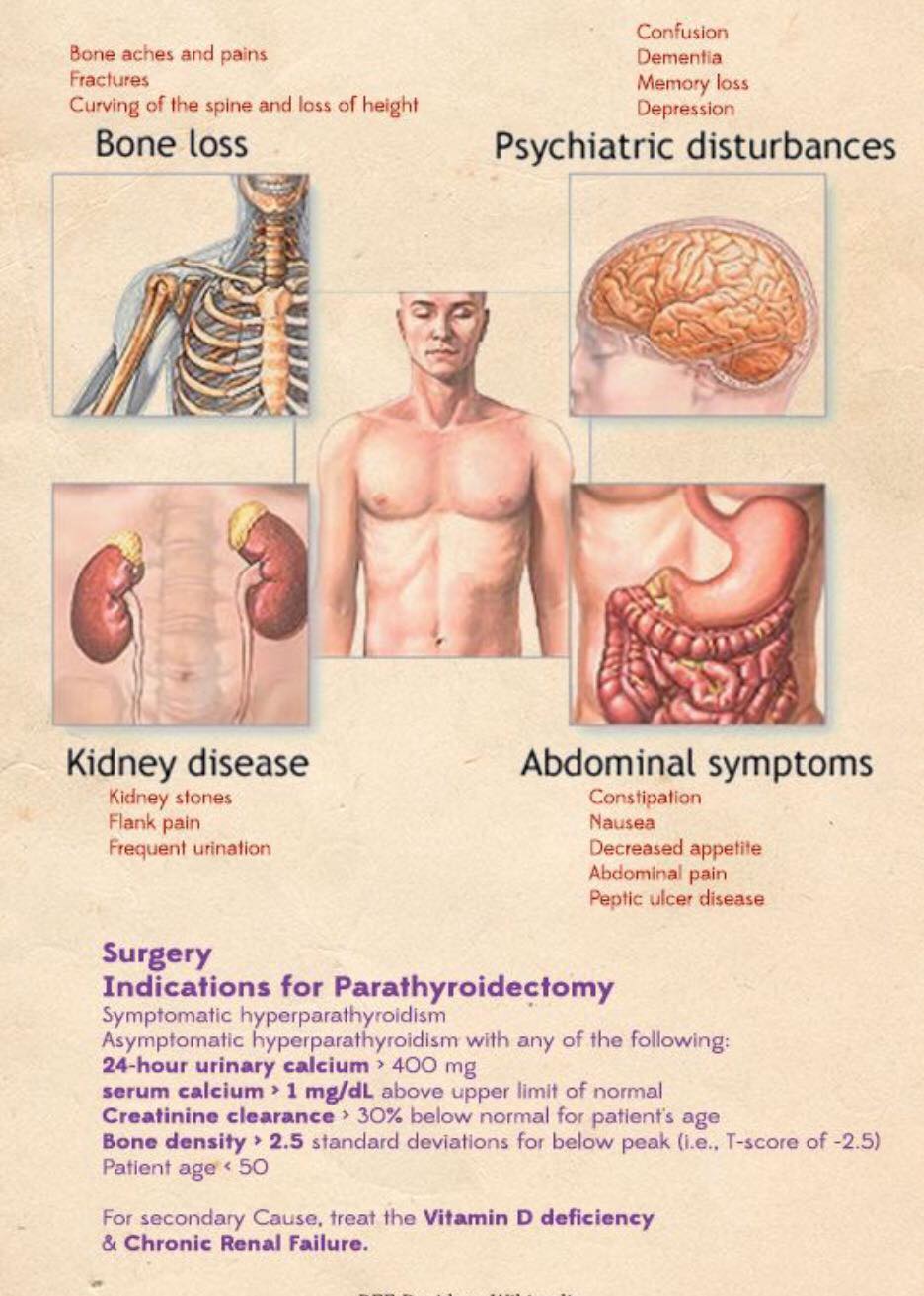
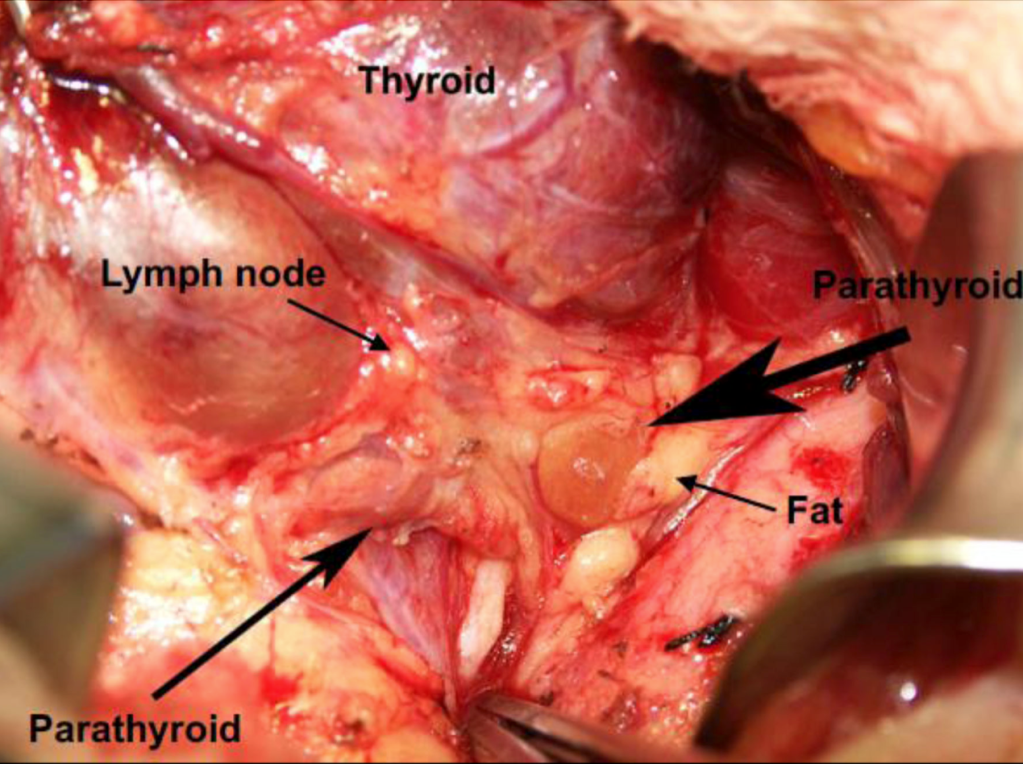
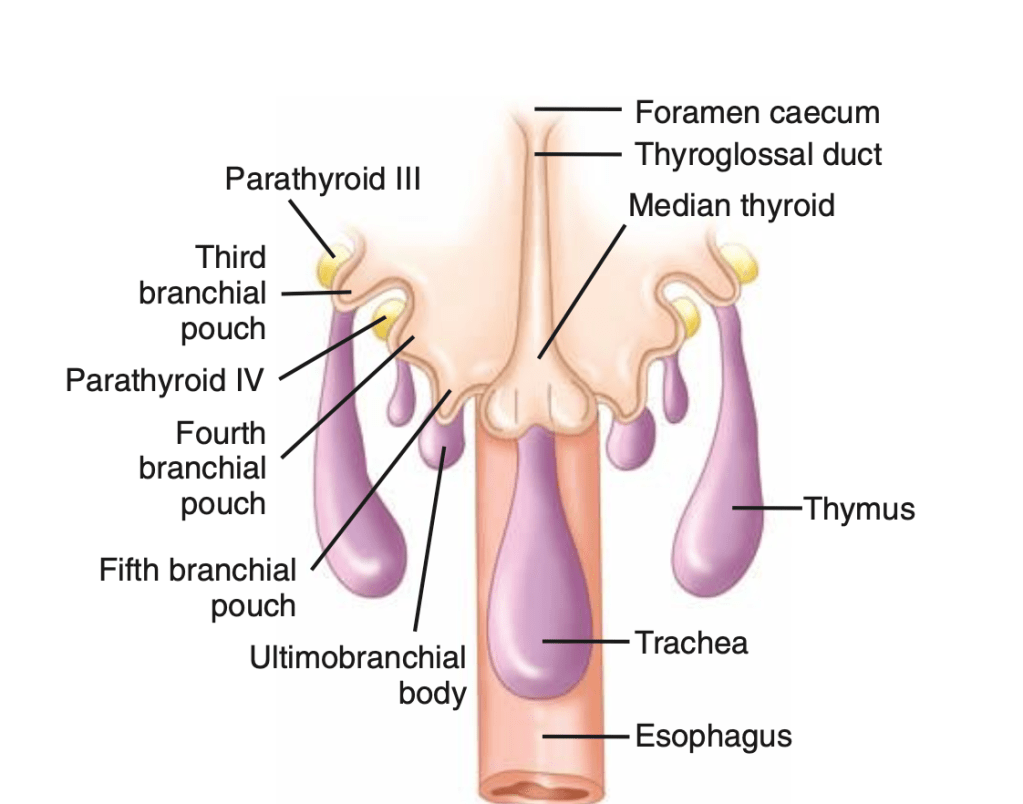
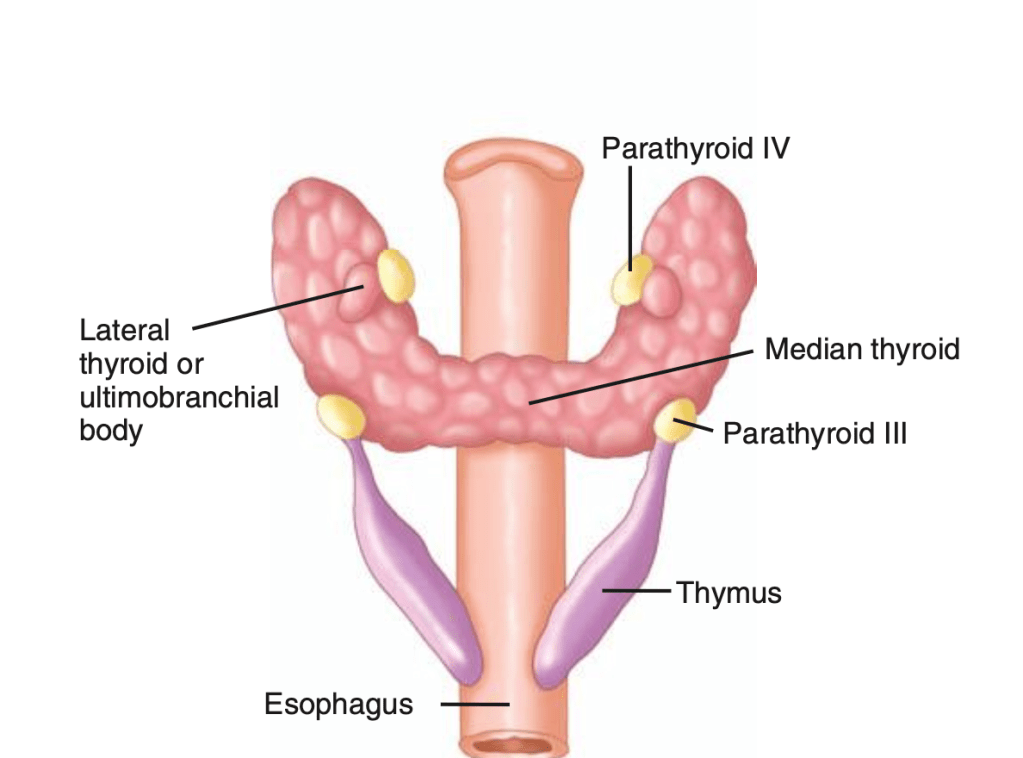
👉The first report for using intraoperative parathyroid hormone (IOPTH) level as an adjunct to guide removal of hyperfunctioning parathyroids was published by Dr. G. Irvin the 3rd et al. in 1993.
👉There are many criteria, however, all require judgement to balance risk of removing multiple glands with risk of recurrent / persistent disease, as outlined in this review.
https://www.sciencedirect.com/science/article/abs/pii/S1521690X19300612?via%3Dihub
Dr. Rodrigo Arrangoiz is a board-certified surgical oncologist who subspecializes in breast cancer and head and neck cancer. Dr. Arrangoiz earned his medical degree at the Anahuac University Medical School in Mexico City, Mexico and graduated Suma Cum Laude. He completed his internship and residency in general surgery at Michigan State University, where he was named chief resident during his fifth year of residency. Dr. Arrangoiz also completed a complex surgical oncology, head and neck fellowship at the Fox Chase Cancer Center in Philadelphia and at the same time he undertook a master’s in science (Clinical Research for Health Care Professionals) at Drexel University in Philadelphia. Dr. Arrangoiz also participated in a two-year global online fellowship in head and neck surgery and oncology through the International Federation of Head and Neck Societies / Memorial Sloan Kettering Cancer Center.
Dr. Arrangoiz has participated in multiple courses and academic congresses as a lecturer and guest professor and has also participated in several publications on topics related to his specialty that include oral cavity cancer, hyperparathyroidism, thyroid cancer, breast cancer, endocrine tumors, squamous cell carcinoma of the head and neck, and more. He is board certified by the American Board of Surgery, the Mexican Board of General Surgery and the Mexican Board of Oncology.
He is a member of various medical associations such as the American College of Surgeons, American Thyroid Association, American Head and Neck Society, American Medical Association, American Society of Clinical Oncology, Association of Academic Surgeons, Society of Surgical Oncology, The Society of Surgery of the Alimentary Tract, Society of American Gastrointestinal Endoscopic Surgeons, and the American Society of Breast Surgeons, among others.
Specialty:
Head and Neck Surgery
Thyroid and Parathyroid Surgery
Breast Surgery
Complex Surgical Oncology
Areas of Clinical Interest:
Malignant thyroid disease (papillary, follicular, medullary, anaplastic thyroid cancer, thyroid lymphoma, and metastatic disease to the thyroid gland) benign thyroid diseases (goiter, multinodular goiter, substernal goiter, hyperthyroidism), hyperparathyroidism / hypercalcemia, benign and malignant breast diseases, head and neck surgery and head and neck cancer.
#Arrangoiz #ParathyroidSurgeon #ParathyroidExpert #Hyperparathyroidism #PrimaryHyperparathyroidism #CancerSurgeon #EndocrineSurgery #Teacher #Surgeon #HeadandNeckSurgeon #SurgicalOncologist #ParathyroidAdenoma #Hypercalcemia #ElevatedCalciumLevels #Miami #MountSinaiMedicalCenter #MSMC #Mexico #Hialeah