- With the advent of taxanes in the 1990’s:
- A series of phase I / II trials used paclitaxel or docetaxel for induction chemotherapy (IC) for head and neck cancer came out
- While taxane and platinum doublets:
- Have not shown outstanding results (Singh et al., 2022):
- Adding docetaxel to cisplatin and 5-FU or cisplatin, 5-FU, and leucovorin:
- Produced response rates exceeding 80% (Monnerat et al., 2002)
- Adding docetaxel to cisplatin and 5-FU or cisplatin, 5-FU, and leucovorin:
- Have not shown outstanding results (Singh et al., 2022):
- Later, several phase 3 trials:
- Caused the three-drug combination TPF:
- Docetaxel 75 mg/m2 IV on day 1, cisplatin 75 to 100 mg/m2 IV on day 1, and 5-fluorouracil 750 to 1000 mg/m2 IV on days 1 to 4 or 5:
- To become the standard regimen for IC:
- This included the cardinal TAX 323 / EORTC 24971 (Vermorken et al., 2007) and TAX 324 (Posner et al., 2007) trials:
- Which demonstrated the superiority of TPF over PF in terms of:
- Progression free survival (PFS)
- Overall survival (OS)
- Local control
- Organ preservation
- Quality of life
- In resectable and unresectable head and neck cancers
- Which demonstrated the superiority of TPF over PF in terms of:
- This included the cardinal TAX 323 / EORTC 24971 (Vermorken et al., 2007) and TAX 324 (Posner et al., 2007) trials:
- To become the standard regimen for IC:
- Docetaxel 75 mg/m2 IV on day 1, cisplatin 75 to 100 mg/m2 IV on day 1, and 5-fluorouracil 750 to 1000 mg/m2 IV on days 1 to 4 or 5:
- Caused the three-drug combination TPF:
- The addition of taxanes to PF induction chemotherapy for patients with stage III or IV disease with no distant metastases:
- Yields superior outcomes compared with PF alone
- The TAX 323 and TAX 324 studies randomly assigned patients to:
- Three cycles of PF versus three cycles of TPF
- In both studies, the total dose per cycle of 5-FU was reduced in the TPF regimens compared with the PF regimens
- TAX 323 / EORTC 24971:
- Was restricted to patients with unresectable disease
- Subtle differences in the dose and schedule of cisplatin and 5-FU existed in the TPF regimens in these two studies
- After induction chemotherapy:
- Patients received definitive radiation therapy alone in TAX 323 or with concurrent weekly carboplatin (area under the curve of 1.5) in TAX 324
- Both studies demonstrated superior outcomes with TPF compared with PF
- In TAX 323:
- Overall response rates after induction chemotherapy:
- 37% versus 26%:
- Were significantly higher for patients treated with TPF versus PF
- 37% versus 26%:
- Overall response rates after induction chemotherapy:
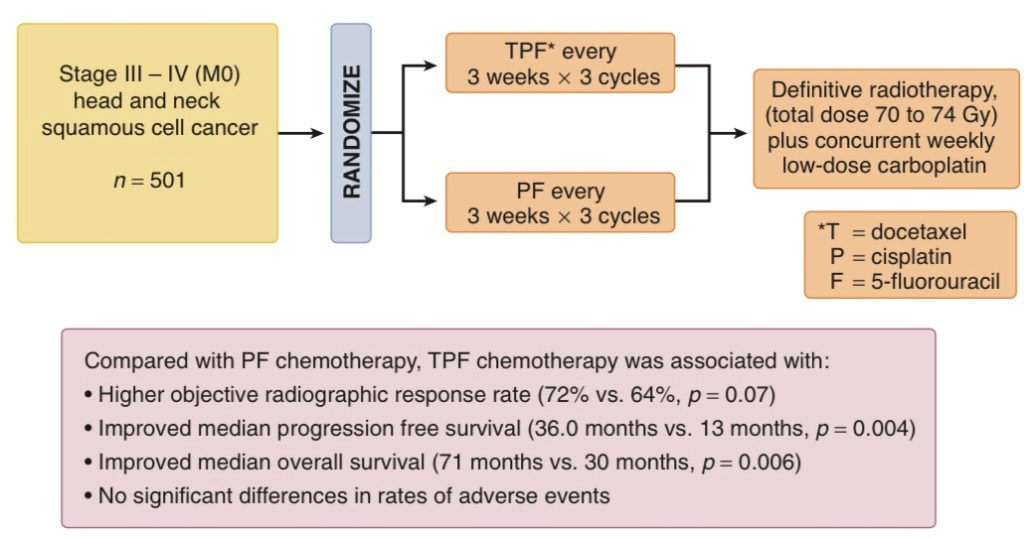
- In TAX 324:
- The overall response rates after induction chemotherapy:
- 72% versus 64%, p = .07
- 3-year overall survival were superior for the TPF group:
- 62% versus 48%
- The overall response rates after induction chemotherapy:
- These results are consistent with those of a randomized trial reported by Hitt and colleagues:
- That evaluated the addition of paclitaxel to cisplatin and 5-FU in patients with stage III or IV disease without distant metastasis:
- The addition of paclitaxel yielded a significant improvement in response rate to induction chemotherapy and a trend toward improvement in overall survival
- That evaluated the addition of paclitaxel to cisplatin and 5-FU in patients with stage III or IV disease without distant metastasis:
- These trials, however, were not designed to compare the strategy of induction chemotherapy followed by chemoradiation versus primary chemoradiation
- Several phase III randomized clinical trials that followed failed to demonstrate a significant efficacy advantage with this sequential approach
- (Cohen et al., 2014; Haddad et al., 2013; Hitt et al., 2014)
- The negative results in the PARADIGM and DeCIDE trials:
- Have been attributed to poor accrual and unexpected favorable outcomes in the control arms (Cohen et al., 2014; Haddad et al., 2013), and the Spanish Head and Neck Cancer Cooperative Group (TTCC) study has only been reported with relatively short follow-up (Hitt et al., 2014)
- The only positive phase III clinical trial demonstrating superior outcomes with TPF induction followed by chemoradiation over chemoradiation alone:
- Was reported in abstract form by Italian investigators from Gruppo di Studio Tumori della Testa e del Collo (GSTTC)
- Taken together, these results suggest that more investigation is required to better elucidate the benefit of induction chemotherapy, and perhaps more importantly better defining the patient population who benefits from the sequential approach
- In Tax 323 TPF was used with a reduced dose form:
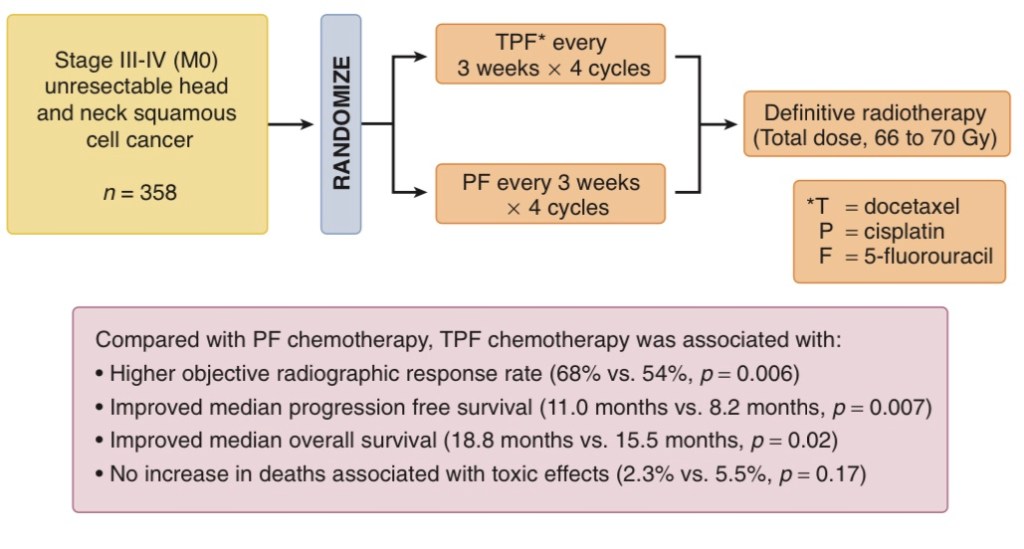
- Nevertheless, the details of TAX trials showed that toxicities were high in both regimens:
- For instance, at least 70% of subjects on TPF and about half of PF patients experienced grade 3 and 4 neutropenia in both trials
- While subjects in TAX 323 received a sequential schedule, that was IC followed by RT rather than CCRT:
- In Tax 324 patients proceeded with carboplatin with RT
- A critical commentary:
- Emphasized on the percentage of patients who were ultimately treated off-protocol in the TPF, and PF arms:
- Which were 21%, and 24% respectively:
- It is assumed that this observation likely reflects greater response rates of taxane-based regimens, rather than the more favorable tolerance of the TPF treatment (Haddad and Posner, 2009)
- Supposedly, some of these subjects of both trials who were not treated with concurrent chemotherapy may have been disadvantaged in receiving alternative treatments
- Also, almost half of the patients in these trials were diagnosed with oropharyngeal cancer which is believed to have a more favorable prognosis, so the results of these trials might not be generalizable (Tural and Kilickap, 2013)
- Which were 21%, and 24% respectively:
- Emphasized on the percentage of patients who were ultimately treated off-protocol in the TPF, and PF arms:
- The update of MACH-NC with the inclusion of taxane trials:
- Showed that the addition of taxane to PF caused:
- A 7.4% rise of OS in favor of TPF
- This showed great promise, however, remarkably, only half of the patients on TPF went through concomitant chemotherapy as planned and about a third did not start RT in the TPF arm
- Additionally, TPF induction was not compared to CCRT in this study (Blanchard et al., 2013)
- Showed that the addition of taxane to PF caused:
- In the most recent 2021 update on MACH-NC with a follow-up of 9.2 years:
- The superiority of CCRT alone over the addition of IC was once again confirmed:
- The OS benefit of 0.83 [0.79;0.86] with an absolute benefit of 6.5% and 3.6% at 5 and 10 years, respectively:
- However, it failed to prove any survival benefit for TPF induction compared to CCRT alone:
- These data are inconsistent with those reported for PF, which had shown superior survival when compared to CCRT (Lacas et al., 2021)
- However, it failed to prove any survival benefit for TPF induction compared to CCRT alone:
- The OS benefit of 0.83 [0.79;0.86] with an absolute benefit of 6.5% and 3.6% at 5 and 10 years, respectively:
- The superiority of CCRT alone over the addition of IC was once again confirmed:
- A secondary finding of the MACH-NC report was rates of locoregional and distant failures per treatment type (Pignon et al., 2009):
- The hazard ratio for death was 0.81 [0.78;0.86] by CCRT and 0.96 for the addition of IC but with an insignificant confidence interval ranging from 0.9 to 1.02
- Their indirect comparison revealed an improvement in both local (HR, 0.74; 95% CI, 0.70–0.79; P = 0.001), and distant failure, (HR, 0.88; 95% CI, 0.77–1.00; P = 0.04):
- In the CRT group, whereas, IC did not affect locoregional control but particularly reduced metastases (HR, 0.73; 95% CI, 0.61–0.88; P = 0.001):
- The impact on reduced tumor dissemination did not cause survival benefit by IC regimens but instead, a higher local control seems to be causative for the significant survival benefit with CCRT alone compared to the addition of IC
- In the CRT group, whereas, IC did not affect locoregional control but particularly reduced metastases (HR, 0.73; 95% CI, 0.61–0.88; P = 0.001):

