- Differentiated thyroid cancer (DTC) includes papillary, follicular, and oncocytic carcinomas:
- Comprising the vast majority (> 90%) of all thyroid cancers
- In the United States:
- It is estimated that there were 44,020 new cases of thyroid cancer in 2024:
- Compared with 37,200 in 2015 when the last American Thyroid Association (ATA) guidelines were published
- It is estimated that there were 44,020 new cases of thyroid cancer in 2024:
- The yearly incidence tripled from 4.9 per 100,000 in 1975 to:
- 14.3 per 100,000 in 2015
- Approximately 25% of the new thyroid cancers diagnosed in 1988 to 1989 were < 1 cm:
- Compared with 39% of the new thyroid cancer diagnoses in 2008 to 2009:
- This shift to earlier detection / diagnosis correlates with the increasing use of neck ultrasonography and other imaging along with the advent of ultrasound-guided fine needle aspiration (FNA)
- Compared with 39% of the new thyroid cancer diagnoses in 2008 to 2009:
- The incidence of thyroid cancer, and particularly small thyroid cancers:
- Has reduced in the United States since 2014:
- This change in incidence trajectory is likely a reflection of the adoption of guidelines’ recommendations from the ATA and other organizations discouraging FNA of small nodules < 1 cm in the absence of abnormal lymph nodes or local invasion:
- Due to the overall outstanding prognosis associated with these tumors and weighed against the potential risks of unnecessary treatment
- This change in incidence trajectory is likely a reflection of the adoption of guidelines’ recommendations from the ATA and other organizations discouraging FNA of small nodules < 1 cm in the absence of abnormal lymph nodes or local invasion:
- Has reduced in the United States since 2014:
- In addition to changes in the management of early-stage thyroid cancer:
- Prior guidelines introduced criteria to enhance initial decision-making and a response framework following interventions to facilitate further management decisions:
- These have been validated since the prior guidelines, enabling adoption in clinical practice
- Prior guidelines introduced criteria to enhance initial decision-making and a response framework following interventions to facilitate further management decisions:
- There have been major advances in understanding the molecular causes of thyroid cancer development and progression that have created newly approved treatment options for subsets of patients:
- Published data in these and other areas require serial updates of existing guidelines to facilitate clinical care
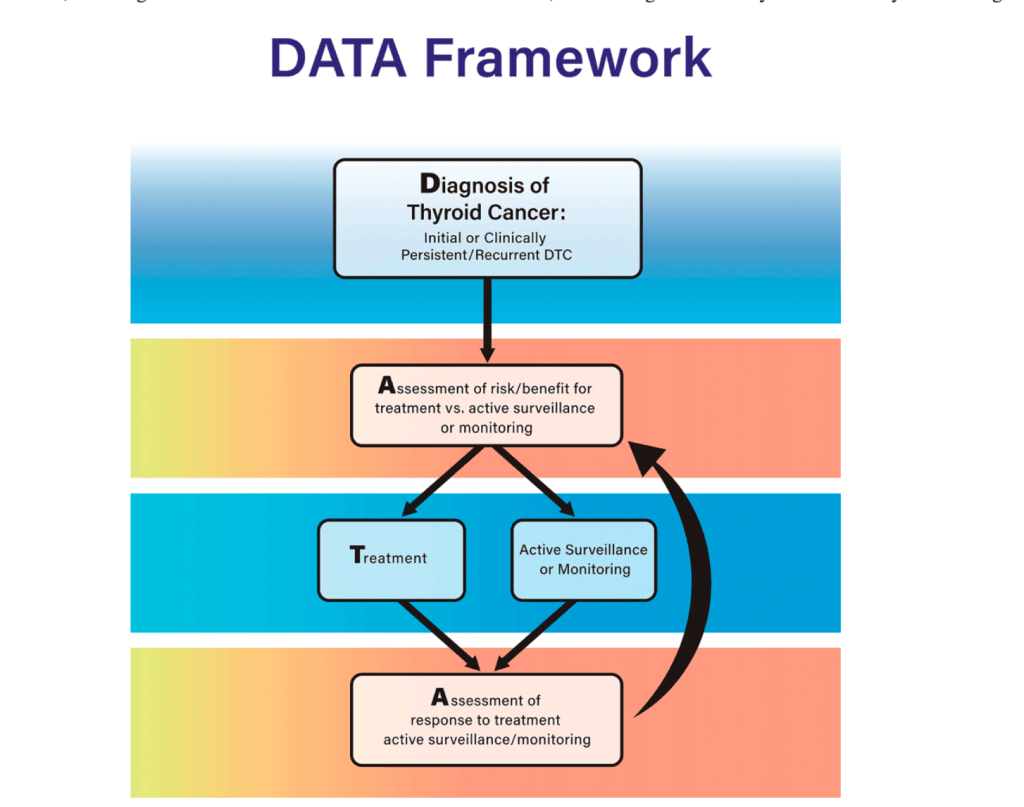
- In the current guidelines, an approach to clinical decision-making is introduced based upon the individual patient and clinician journey with thyroid cancer:
- Which they term DATA:
- Diagnosis
- Risk / benefit Assessment
- Treatment decisions
- Response Assessment
- Which they term DATA:
- This approach begins at the initial diagnosis of thyroid cancer, the diagnosis of residual disease or a clinical recurrence:
- It includes assessment to determine whether a particular intervention is appropriate based on risks and benefits as well as individual patient factors:
- When multiple possible management strategies are available, the framework supports identification of the best treatment option
- It includes assessment to determine whether a particular intervention is appropriate based on risks and benefits as well as individual patient factors:
- Then, after intervention, an assessment of response using the 2025 ATA risk assessment tool is deployed to determine whether more treatment or monitoring is appropriate
- The clinician and the patient can use this DATA framework to help make clinical decisions from diagnosis through the patient’s entire disease course.
- In 1996, the ATA published treatment guidelines for patients with thyroid nodules and DTC:
- Over the last 25 to 30 years, there have been remarkable advances in knowledge affecting the diagnosis and treatment of DTC, but clinical controversy continues to exist in many areas
- In the end, the goal is to provide individualized therapy for each patient based on the best application of clinical data to their unique case:
- For example, a less aggressive approach would be recommended for individuals with early stage DTC who have an excellent prognosis or for individuals at higher risk of side effects, while a more aggressive approach would be recommended for those patients with higher risk disease or those with inadequate response to initial therapy

