- The CheckMate 238 trial:
- Is a pivotal Phase III clinical study evaluating the efficacy of nivolumab (Opdivo) versus ipilimumab (Yervoy):
- As adjuvant therapy for patients with resected stage IIIB, IIIC, or IV melanoma
- Is a pivotal Phase III clinical study evaluating the efficacy of nivolumab (Opdivo) versus ipilimumab (Yervoy):
- The trial demonstrated that adjuvant nivolumab significantly improved recurrence-free survival compared to ipilimumab, with a more favorable safety profile
- This benefit was sustained at 4 and 5 years:
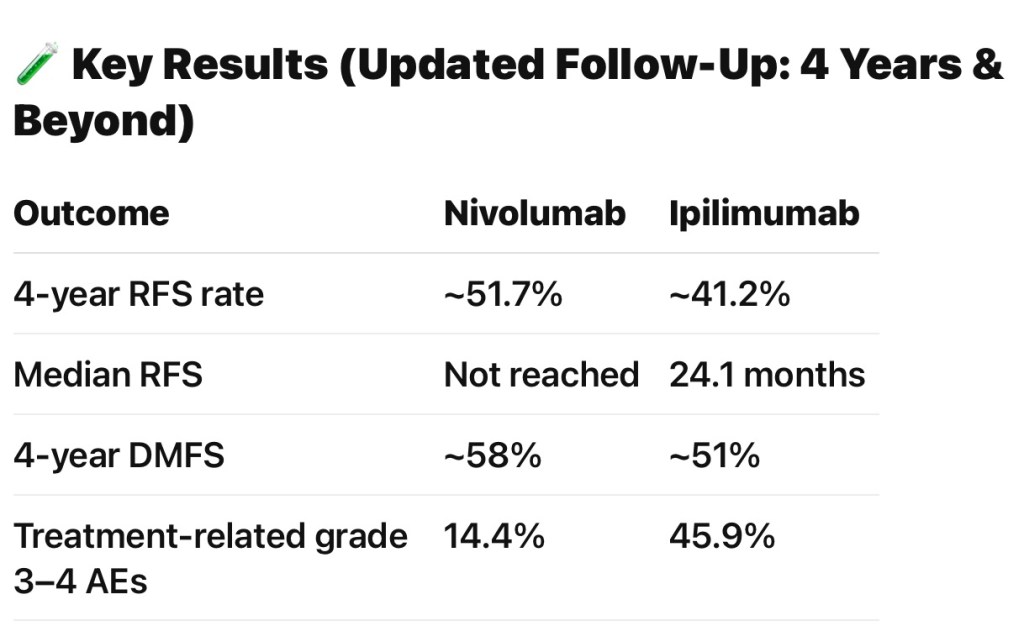
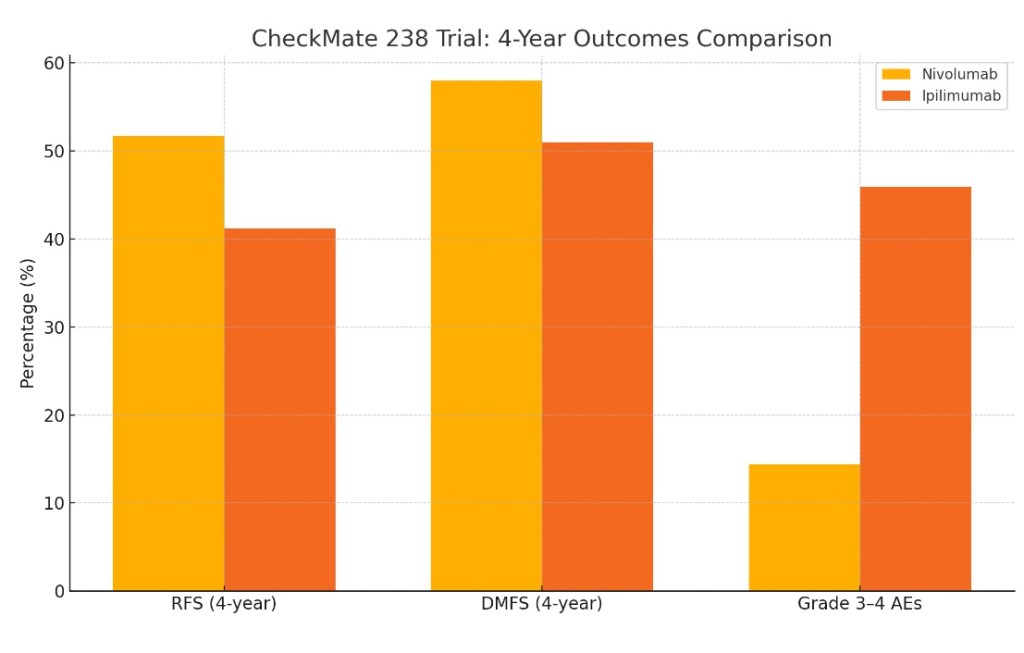
- 4-year RFS rates of 51.7% for nivolumab versus 41.2% for ipilimumab (HR 0.71, 95% CI 0.60–0.86)
- 5-year RFS rates of 50% versus 39%
- Distant metastasis-free survival and overall survival were also numerically higher with nivolumab:
- Though the OS difference was not statistically significant at the time of reporting
- Importantly, nivolumab was associated with a much lower rate of grade 3 to 4 adverse events:
- 14.4% vs. 45.9% for ipilimumab:
- Fewer treatment discontinuations due to toxicity
- 14.4% vs. 45.9% for ipilimumab:
- In the context of local recurrence after adjuvant therapy:
- Post hoc analyses from CheckMate 238 indicate that patients who recur early (≤ 12 months) after adjuvant nivolumab:
- Have limited benefit from anti–PD-1 monotherapy as subsequent systemic therapy:
- But may derive greater benefit from ipilimumab-based regimens or targeted therapy if BRAF-mutant
- Have limited benefit from anti–PD-1 monotherapy as subsequent systemic therapy:
- Patients with late recurrence (> 12 months):
- May still respond to anti–PD-1 rechallenge
- Thus, the trial not only established nivolumab as a standard adjuvant therapy for resected stage III / IV melanoma but also informs management strategies for local recurrence after prior immune checkpoint blockade
- Post hoc analyses from CheckMate 238 indicate that patients who recur early (≤ 12 months) after adjuvant nivolumab:
- Trial Summary:
- Name: CheckMate 238 (NCT02388906)
- Design:
- Randomized, double-blind, multicenter phase III trial
- Population:
- 906 patients with completely resected stage IIIB, IIIC, or IV cutaneous melanoma (AJCC 7th edition)
- Intervention:
- Nivolumab 3 mg/kg IV q 2 weeks
- Ipilimumab 10 mg/kg IV q 3 weeks × 4, then q 12 weeks (every three months)
- Both for up to one year or until recurrence or unacceptable toxicity
- Primary Endpoint:
- Recurrence-free survival (RFS)
- Secondary Endpoints:
- Overall survival (OS)
- Distant metastasis-free survival (DMFS)
- Safety
- Conclusions:
- Nivolumab significantly improved RFS compared with ipilimumab
- Better safety profile for nivolumab:
- Substantially fewer high-grade adverse events
- Nivolumab became a standard adjuvant treatment for resected high-risk melanoma based on these findings
- References:
- Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. Weber J, Mandala M, Del Vecchio M, et al. The New England Journal of Medicine. 2017;377(19):1824-1835. doi:10.1056/NEJMoa1709030.
- Adjuvant Nivolumab Versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results From CheckMate 238. Larkin J, Del Vecchio M, Mandalá M, et al. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2023;29(17):3352-3361. doi:10.1158/1078-0432.CCR-22-3145.
- Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB-C and Stage IV Melanoma (CheckMate 238): 4-Year Results From a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Ascierto PA, Del Vecchio M, Mandalá M, et al. The Lancet. Oncology. 2020;21(11):1465-1477. doi:10.1016/S1470-2045(20)30494-0.
- Outcomes With Postrecurrence Systemic Therapy Following Adjuvant Checkpoint Inhibitor Treatment for Resected Melanoma in CheckMate 238. Weber J, Del Vecchio M, Mandalá M, et al. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2024;42(31):3702-3712. doi:10.1200/JCO.23.01448.
- Recent Advances in the Treatment of Melanoma. Curti BD, Faries MB. The New England Journal of Medicine. 2021;384(23):2229-2240. doi:10.1056/NEJMra2034861.

