- The basic functional unit of the thyroid gland:
- Is the thyroid follicle:
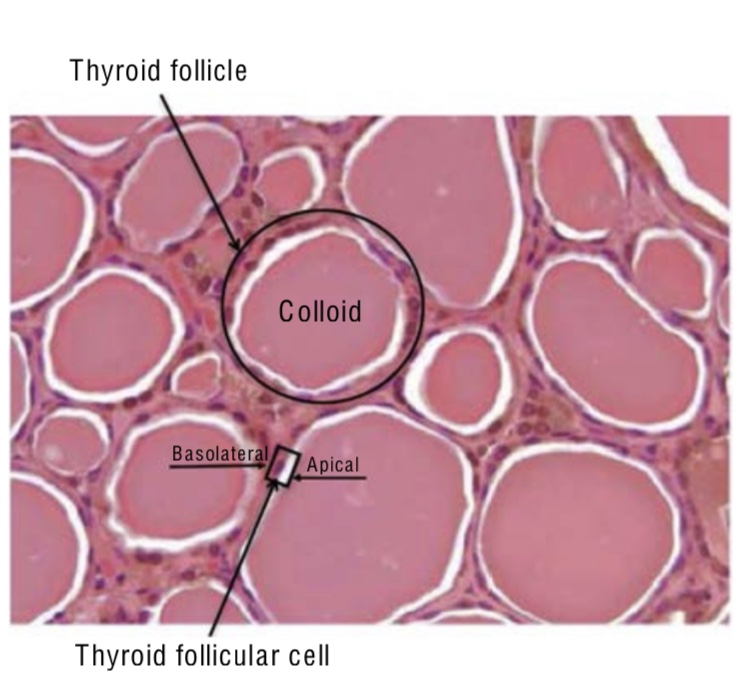
- The thyroid follicle contains a single layer of thyroid follicular cells (epithelial cells):
- That form a sphere with a follicular lumen:
- Which is filled with a colloid protein aggregate
- That form a sphere with a follicular lumen:
- Thyroid follicular cells are polar:
- The apical membrane is adjacent to the follicular lumen
- The basolateral membrane is the one in contact with capillaries and the circulatory system (Figure)
- The thyroid follicle contains a single layer of thyroid follicular cells (epithelial cells):
- Is the thyroid follicle:
- Thyroid hormone synthesis:
- Is activated by the binding of thyroid-stimulating hormone (TSH) to the TSH receptor on the basolateral membrane:
- Which activates adenylate (adenylyl) cyclase and increases intracellular cyclic adenosine monophosphate (cAMP):
- Leading to phosphorylation of protein kinase A and activation of targets in the cytosol and nucleus of the thryoid cell:
- Through this cAMP pathway, TSH stimulates the accumulation of iodide in the thyroid
- This initiates the cascade that results in thyroid hormone synthesis and secretion:
- Which includes iodide transport, synthesis of thyroglobulin, iodination of thyroglobulin, and secretion of the thyroid hormones (Figure)
- Is activated by the binding of thyroid-stimulating hormone (TSH) to the TSH receptor on the basolateral membrane:

- After the binding of TSH:
- The initial step in the thyroid hormone synthesis pathway:
- Is iodide transport across the basolateral membrane of the thryoid follicular cell:
- Mediated by the Na+/I (NIS) symporter
- Is iodide transport across the basolateral membrane of the thryoid follicular cell:
- The initial step in the thyroid hormone synthesis pathway:
- NIS is a sodium-dependent transporter:
- So iodine is only transported with an inward sodium gradient:
- Which is in turn maintained by the action of the Na-K-ATPase
- So iodine is only transported with an inward sodium gradient:
- The intracellularly accumulated iodide ion is then passively translocated across the apical membrane into the colloid protein aggregate:
- Via pendrin proteins and Cl- channels
- The transported (effluxed) iodide ion becomes covalently attached to the precursor thyroid hormone glycoprotein:
- Thyroglobulin:
- At the interface between the apical membrane and the follicular lumen by the enzyme:
- Thyroperoxidase (TPO)
- At the interface between the apical membrane and the follicular lumen by the enzyme:
- Thyroglobulin:
- Further iodinization (organification) of tyrosine molecules on the thyroglobulin glycoprotein:
- Then occurs via TPO facilitating the further incorporation of iodide onto the tyrosine residues:
- Tyrosine molecules (thyrosyl residues) in the thyroglobulin molecule:
- Are then iodinated to form:
- Monoiodotyrosines (MITs) and diiodotyrosines (DITs) (Figure)
- Incorporation of iodide into protein is referred to as:
- Organification
- Are then iodinated to form:
- Tyrosine molecules (thyrosyl residues) in the thyroglobulin molecule:
- Then occurs via TPO facilitating the further incorporation of iodide onto the tyrosine residues:
- It should be noted that this process of oxidation of iodide, organification, and coupling is dependent on:
- The presence of hydrogen peroxide present intralumenally and truly occurs simultaneously
- The bioactive thyroid hormones:
- L-thyroxine / tetraiodothyronine (T4) and triiodothyronine (T3):
- Are formed by the coupling of two DITs or one DIT with one MIT, respectively:
- By TPO (Figure)
- Are formed by the coupling of two DITs or one DIT with one MIT, respectively:
- L-thyroxine / tetraiodothyronine (T4) and triiodothyronine (T3):
- T4 and T3 remain attached to thyroglobulin and are stored as colloid within the follicular lumen:
- Where they remain available for release through TSH stimulation
- In healthy and iodine-sufficient individuals:
- The majority of thyroid hormone in colloid is stored as:
- T4 with a small amount (~ 20%) stored as T3
- The majority of thyroid hormone in colloid is stored as:
- Upon stimulation of the TSH receptor:
- A cytoplasmic vesicle is formed for uptake of colloid into the follicular cell through pinocytosis (micropinocytosis) (Figure)
- The cytoplasmic vesicles fuse with lysosomes:
- Forming phagolysosomes (intracellularly):
- In which Tg is broken down by proteolysis:
- Proteases hydrolyze the peptide bonds of thyroglobulin:
- To release T4 and T3 into the cytoplasm
- Proteases hydrolyze the peptide bonds of thyroglobulin:
- The thyroid hormone transporter:
- Monocarboxylate transporter 8 (MCT8):
- Located in the basolateral membrane of the thyroid follicular cell:
- Is expressed in the thyroid gland and is important for transport of T4 and T3 out of the thyroid gland and into the circulation
- Located in the basolateral membrane of the thyroid follicular cell:
- Production of thyroid hormone varies widely between:
- 75 and 250 mcg daily
- Monocarboxylate transporter 8 (MCT8):
- In which Tg is broken down by proteolysis:
- Forming phagolysosomes (intracellularly):
- In the blood:
- Approximately 99.97% of T4 and 99.7% of T3 are bound to the binding proteins:
- Thyroxine binding globulin (TBG), transthyretin (also known as prealbumin), and albumin:
- Of these, TBG has the highest affinity to bind thyroid hormone:
- Binding approximately 75% of both T4 and T3 in circulation) and is the most clinically relevant among the binding proteins
- Transthyretin, previously referred to as prealbumin:
- Binds approximately 20% of the circulating T4 and < 5% of T3
- Albumin has the lowest affinity for thyroid hormone, but is the most abundant of the proteins:
- Binds 5% of the T4 and 20% of the T3
- Of these, TBG has the highest affinity to bind thyroid hormone:
- Thyroxine binding globulin (TBG), transthyretin (also known as prealbumin), and albumin:
- Approximately 99.97% of T4 and 99.7% of T3 are bound to the binding proteins:
- In total, most of the thyroid hormones in circulation are in the bound state and biologically inactive:
- The unbound thyroid hormones:
- Free T4 (0.03%) and free T3 (0.3%):
- Enter the target cells
- Free T4 (0.03%) and free T3 (0.3%):
- In some tissues, such as those from the brain and pituitary:
- Specific thyroid hormone membrane transporters are required for thyroid hormone uptake:
- Principally monocarboxylate transporter 8 (MCT8)
- Specific thyroid hormone membrane transporters are required for thyroid hormone uptake:
- The unbound thyroid hormones:
- Triiodothyronine / T3:
- Binds with a much greater affinity to the thyroid hormone receptors and for a longer period of time:
- Compared with T4
- T3 is regarded as the primary active thyroid hormone
- Binds with a much greater affinity to the thyroid hormone receptors and for a longer period of time:
- Tetraiodothyronine / T4:
- Is synthesized exclusively by the thyroid gland:
- Whereas T3 is produced primarily in peripheral tissues:
- From the deiodination of circulating T4
- Only about 20% of the daily T3 requirement:
- Is synthesized directly by the thyroid gland
- Whereas T3 is produced primarily in peripheral tissues:
- Is synthesized exclusively by the thyroid gland:
- The activation of T4 to T3 requires the 5’-deiodinase enzymes type 1 (Dio1) and type 2 (Dio2):
- These enzymes are differentially expressed:
- Dio1 predominantly in the liver
- Dio2 in tissues that require local T3 production, such as:
- The brain, pituitary, muscle, and brown fat
- These enzymes are differentially expressed:
- In the setting of fluctuating T4 levels:
- Deiodinase activity is modulated to maintain normal circulating and target tissue T3 levels (Figure)

- When serum T4 levels fall, as in hypothyroidism:
- Dio2 is activated locally by a deubiquitination process:
- That reduces Dio2 degradation:
- Increases Dio2 activity, and promotes greater conversion of T4 to the bioactive T3:
- Normal serum T3 levels are maintained until the serum T4 becomes very low
- Increases Dio2 activity, and promotes greater conversion of T4 to the bioactive T3:
- That reduces Dio2 degradation:
- Dio2 is activated locally by a deubiquitination process:
- Thyroid metabolism is influenced by illness and drugs:
- The activity of Dio1 and the resulting T3 level is reduced in:
- Malnutrition
- Critical illness
- By the action of certain medications:
- Beta-blockers
- Ipodate
- Amiodarone
- Dexamethasone
- Propylthiouracil
- The activity of Dio1 and the resulting T3 level is reduced in:
- During starvation and acute illness:
- Expression of the 5 deiodinase type 3 (Dio3) is increased and converts the bioactive T4 and T3:
- To two biologically inactive molecules:
- Reverse T3 (rT3) and 3,3’diiodothyronine (T2)
- To two biologically inactive molecules:
- Expression of the 5 deiodinase type 3 (Dio3) is increased and converts the bioactive T4 and T3:
- The available free T3:
- Binds to a nuclear thyroid hormone receptor at the target tissue:
- Alters gene expression, and regulates cellular function (Figure)
- Binds to a nuclear thyroid hormone receptor at the target tissue:

- The thyroid hormone nuclear receptor (THR) is a protein within a superfamily of receptors:
- That bind steroid and steroid-like hormones such as retinoic acid, vitamin D, and estrogen
- The THRs mediate the majority of biologic activities of T3:
- Two THR genes, alpha and beta:
- Encode four THR isoforms:
- Alpha 1, beta 1, beta 2, and beta 3
- Encode four THR isoforms:
- The transcriptional activity of THRs is regulated by the binding of T3:
- The thyroid hormone response elements located on the promoters of the T3 regulated gene, by the developmental- and tissue-dependent expression of THR isoforms and by nuclear cofactors or coregulatory proteins
- There are also nongenomic actions of iodothyronine (T4) that are not mediated by intranuclear THR:
- Action at the plasma membrane is mediated by the integrin alpha-v beta 3 receptor that binds T4, and activates ERK1/2, which leads to changes in membrane ion transport, such as the Na(+)/H(+) exchanger, and is also involved in other important cellular events such as cell proliferation
- Two THR genes, alpha and beta:

