- The second most common type of thyroid cancer:
- Is follicular thyroid carcinoma (FTC):
- 4.6% of the cases based on the SEER database between 2010 and 2014
- Is follicular thyroid carcinoma (FTC):
- Fundamentally all follicular carcinomas are:
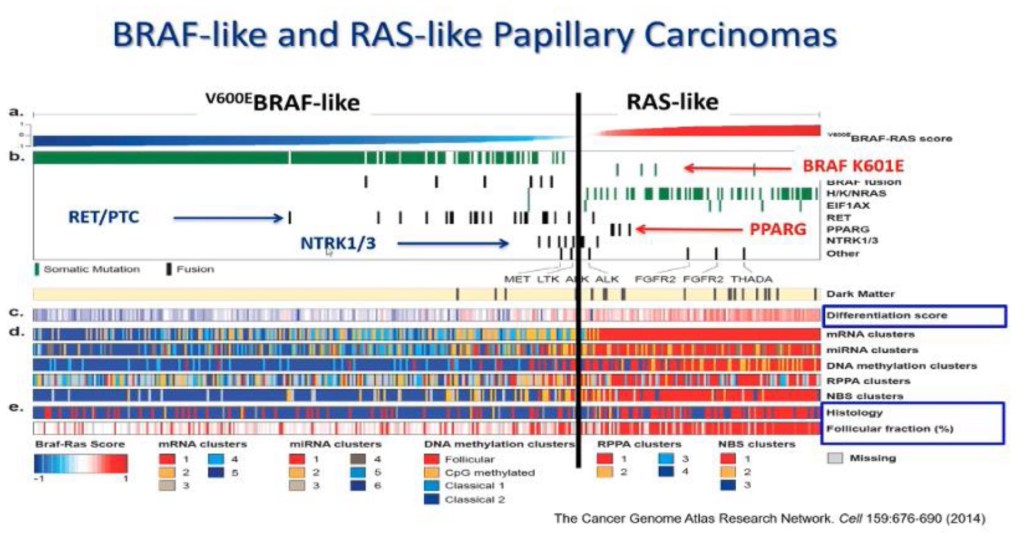
- RAS-like tumors:
- There profile is different from classic PTC because they do not have BRAF mutations:
- Most of them have RAS and RAS-like mutations
- There profile is different from classic PTC because they do not have BRAF mutations:
- Yoo S.K et al, from Korea showed that the genetic profile of follicular carcinomas:
- Is very similar to follicular adenomas (FA) because they are related tumors:
- Most FTC originate from a FA and eventually break through the capsule and become carcinomas
- Is very similar to follicular adenomas (FA) because they are related tumors:
- Encapsulated follicular variant of PTC:
- Their molecular profile is much closer to a FA and FTC than to classic PTC
- Infiltrative follicular variant of PTC has a molecular profile:
- That is more like classic PTC than FTC
- The biologic difference between
follicular pattern RAS-like tumors and classic PTC:- Is the infiltrative growth pattern (Figure)
- The difference between these tumors is not only phenotypically based on gross pattern, but also based on biological and clinical differences:
- Because follicular pattern RAS-like tumors:
- Retain avidity to radioactive iodine
- BRAF-like tumors (classic PTC and infiltrative follicular variant of PTC) have the classic features of PTC:
- They are infiltrative, they spread to lymph nodes first and later to distant sites, and they lose the expression of genes associated with thyroid differentiation
- RAS-like tumors (FA, FTC, NIFTP, and invasive encapsulated follicular variant of PTC):
- May or may not have nuclear features of PTC, they are encapsulated, they spread to distant sites (rarely to lymph nodes), and they retain expression of genes associated with thyroid differentiation
- Because follicular pattern RAS-like tumors:
- RAS-like tumors:
- The RAS genes (HRAS, KRAS and NRAS):
- Encode for the interconnected G-proteins:
- That play a critical role in the intracellular transduction of signals arising from cell membrane receptors
- Encode for the interconnected G-proteins:
- RAS protein in its inactive state:
- Is bound to guanosine diphosphate (GDP):
- Upon activation, it releases GDP and binds guanosine triphosphate (GTP):
- Thus activating the MAPK and other signaling pathways, such as PI3K/AKT
- Upon activation, it releases GDP and binds guanosine triphosphate (GTP):
- Typically, the activated RAS / GTP protein becomes promptly inactive:
- Due to its intrinsic GTPase activity and the action of cytoplasmic GTPase-activating proteins:
- Point mutations in the domains of the RAS gene either increase its affinity for GTP (mutations in codons 12 and 13) or inactivate its autocatalytic GTPase activity (mutation in codon 61):
- The consequence of this is that the mutant protein becomes permanently switched in the active position and continuously activates its downstream targets
- Point mutations in the domains of the RAS gene either increase its affinity for GTP (mutations in codons 12 and 13) or inactivate its autocatalytic GTPase activity (mutation in codon 61):
- Due to its intrinsic GTPase activity and the action of cytoplasmic GTPase-activating proteins:
- Mutations in the RAS genes are believed play
an early role in the cellular transformation and may predispose to the progression benign tumors to malignant tumors - Point mutations involving the specific sites (codons 12, 13 and 61) of the NRAS, HRAS or KRAS genes:
- Are identified in roughly 10% to 20% of PTC:
- PTC holding RAS mutation invariably have follicular subtype histology:
- This mutation also correlates with significantly less prominent nuclear features of PTC, more common
thyroid encapsulation, and a lower rate of lymph node metastases
- This mutation also correlates with significantly less prominent nuclear features of PTC, more common
- A few studies have linked RAS
mutations with PTC:- That have a more aggressive behavior, such as a higher frequency of distant metastases
- Mutations in the RAS gene are not limited to PTC and also found in other benign and malignant thyroid neoplasms, as well as in tumors from other tissues
- RAS gene mutations are identified in approximately:
- 20% to 40% of follicular thyroid adenomas (FTA)
- 40% to 50% of FTC
- 20% to 40% of anaplastic thyroid carcinoma (ATC)
- PTC holding RAS mutation invariably have follicular subtype histology:
- When a FNA cytology is indetermined and the molecular profile identifies a RAS mutation:
- The risk of malignancy varies
between the type of mutation:- HRAS = 71%
- NRAS = 63%
- KRAS = 33%
- The risk of malignancy varies
- Are identified in roughly 10% to 20% of PTC:
- Is bound to guanosine diphosphate (GDP):
- The concept of progression from benign to malignant tumors is supported by the molecular profile shared by these different tumors:
- More evidence supporting this concept of cancer progression is the similar morphology that these lesions have, along with experimental mouse data showing very similar results
- Pathologist observing thyroid nodules have detected this step wise progression
- The nodule in Figure developed from a single cell driven by the RAS mutation, it continued to grow and grow, it eventually forms a capsule, looking microscopically like a benign adenoma (goiter), then it continues to progress, it accumulates more genetic alterations (micro mRNA, and it involves other molecular pathways), it becomes a tumor, it eventually breaks through the capsule, and it will give you invasive encapsulated follicular variant of PTC:
- If an FNA is performed of area A it would come back as Bethesda II, in area B it would come back as a Bethesda IV, and in area C as Bethesda V:
- This has changed the practice of cytopathology with molecular
testing helping us understand better the biological nature of these tumors
- This has changed the practice of cytopathology with molecular
- If an FNA is performed of area A it would come back as Bethesda II, in area B it would come back as a Bethesda IV, and in area C as Bethesda V:

Figure: Molecular Changes Precede Histological Changes. The tumor measures 2.5 cm, it has a thin
capsule (black arrows) and shows an area of flat epithelial cells lining the follicles representing a benign thyroid
goiter (tumor area A). Microfollicular areas with well-developed nuclear features of PTC are seen in tumor area
B representing a probable NFTP. Tumor area C has separation artifact with the formation of papillary structures
with nuclear features of PTC. Molecular studies of each section will show NRAS mutation.

