- Papillary thyroid carcinoma (PTC):
- Is one of the best molecularly understood cancers:
- With more that 97% of the driver mutations identified
- Is one of the best molecularly understood cancers:
- One of the main goals of the cancer genome atlas research network study (TCGA) thyroid project:
- Was to detect cancer-initiating mutations, i.e., driver mutations:
- In those cases that lacked the well-known PTC driver mutations (BRAF V600E, point mutations of RAS genes, and gene fusions involving RET and NTRK):
- These cases are referred to by computational biologists as “dark matter” cases
- In those cases that lacked the well-known PTC driver mutations (BRAF V600E, point mutations of RAS genes, and gene fusions involving RET and NTRK):
- These very important studies under the umbrella of NCI and NIH analyzed 496 PTCs that permitted several analyses:
- That showed that approximately 75% of all PTC:
- Developed through the molecular mechanism of point mutation:
- The most common been the:
- BRAF V600E mutation
- The second most common the:
- RAS mutation
- The thyroid most common the:
- TERT mutation – That constituted a small percentage of tumors but was found to be a marker of aggressive disease
- Roughly 15% of PTC develop through the mechanism of gene fusions:
- The most common been RET / PTC,
NTRK 1/3, ALK, BRAF, PAX8 / PPARG - The RET / PTC, NTRK 1/3, ALK have very important therapeutic implications for advanced thyroid cancer:
- Because of the availability of targeted inhibitors with low toxicity and
high efficacy for the management of these tumors
- Because of the availability of targeted inhibitors with low toxicity and
- The most common been RET / PTC,
- Roughly 7% of PTC develop exclusively from the molecular mechanism of copy number alterations (CNA) (Figure)
- That showed that approximately 75% of all PTC:
- Was to detect cancer-initiating mutations, i.e., driver mutations:


- Roughly 45% to 75% of PTC (29% to 83%) have a mutation in the BRAF gene:
- Making it the most frequently known genetic
alteration in PTC - Practically all mutations involve:
- Nucleotide 1799 and result in a valine-to-glutamate substitution at residue 600 (V600E):
- This point mutation leads to constitutive activation of BRAF kinase:
- Resulting in a constant phosphorylation of MEK and downstream effectors of the MAPK pathway (Figure)
- This point mutation leads to constitutive activation of BRAF kinase:
- Other mechanisms of BRAF activation in PTC include:
- K601E point mutation:
- Small in-frame insertions or deletions surrounding codon 600
- AKAP9-BRAF rearrangement:
- Which is more common in PTC associated with radiation exposure
- K601E point mutation:
- Nucleotide 1799 and result in a valine-to-glutamate substitution at residue 600 (V600E):
- Making it the most frequently known genetic
- BRAF V600E mutation:
- Is the prevailing mutation in PTC:
- With classical histology and in the tall cell subtype:
- But is rare in follicular subtypes tumors
- With classical histology and in the tall cell subtype:
- In multiple studies, the existence of the BRAF mutation:
- Has been linked with aggressive tumor biology such as:
- Advanced stage at presentation
- Extrathyroidal extension (infiltrative)
- Recurrence
- Lymph node or distant metastases
- Has been linked with aggressive tumor biology such as:
- BRAF V600E mutation:
- Is an independent predictor of tumor recurrence:
- Even in patients with stage I to stage II disease
- Is an independent predictor of tumor recurrence:
- The ability of thyroid cancers to trap
radioiodine:- Has been identified to be decreased in tumors with the BRAF V600E mutations and this may lead to treatment failures of the recurrent disease:
- Which is due to the dysregulation of the function of sodium iodide
symporter (NIS) and other genes metabolizing iodide in the thyroid follicular cells
- Which is due to the dysregulation of the function of sodium iodide
- Has been identified to be decreased in tumors with the BRAF V600E mutations and this may lead to treatment failures of the recurrent disease:
- In thyroid nodules, the V600E BRAF mutation is limited to PTC, poorly differentiated and ATC arising from PTC:
- Consequently, the identification of the BRAF V600E mutation in FNA cytology of thyroid nodules:
- Practically confirms the diagnosis of PTC
- Testing for the BRAF mutation is very useful diagnostically in thyroid FNA samples with indeterminate results:
- As it can help to determine the diagnosis of PTC in a important portion of these biopsies
- Consequently, the identification of the BRAF V600E mutation in FNA cytology of thyroid nodules:
- Is the prevailing mutation in PTC:
- Pathologically PTC is one disease and biologically at a minimum PTC has two significantly different groups:
- Based on multiple type of analysis like gene expression alterations, microRNA alterations, DNA methylation, transcriptomics (Figure)
- The Cancer Genome Atlas (TCGA) study on PTC highlighted that PTC can be grouped into:
- BRAFV600E-like and RAS-like tumors:
- BRAFV600E-like tumors:
- Also harbor RET fusions
- RAS-like tumors:
- Show RAS mutations, the BRAFK601E, and PPARG and THADA fusions
- BRAFV600E-like tumors:
- The V600E BRAF–like PTC:
- Are usually classic PTC and tall cell subtype of PTC
- Clinically when we look at the differentiation score:
- Which is the expression of genes involved in iodine metabolism and synthesis:
- BRAF tumors:
- Loose expression of thyroid differentiation markers
- RAS-like tumors retain them:
- Almost at the level of a normal thyroid cell
- This is important therapeutically because one of the most efficient management options in thyroid cancer is iodine therapy
- RAS-like tumors:
- Are usually follicular variant PTC
- BRAF tumors:
- Usually all BRAF mutations are BRAF-like and all RAS mutations are RAS-like:
- But all other type of molecular alterations can be hidden in either BRAF-like and RAS-like:
- For example, BRAF K601E mutations are found in RAS-like tumors
- But all other type of molecular alterations can be hidden in either BRAF-like and RAS-like:
- Which is the expression of genes involved in iodine metabolism and synthesis:
- BRAFV600E-like and RAS-like tumors:
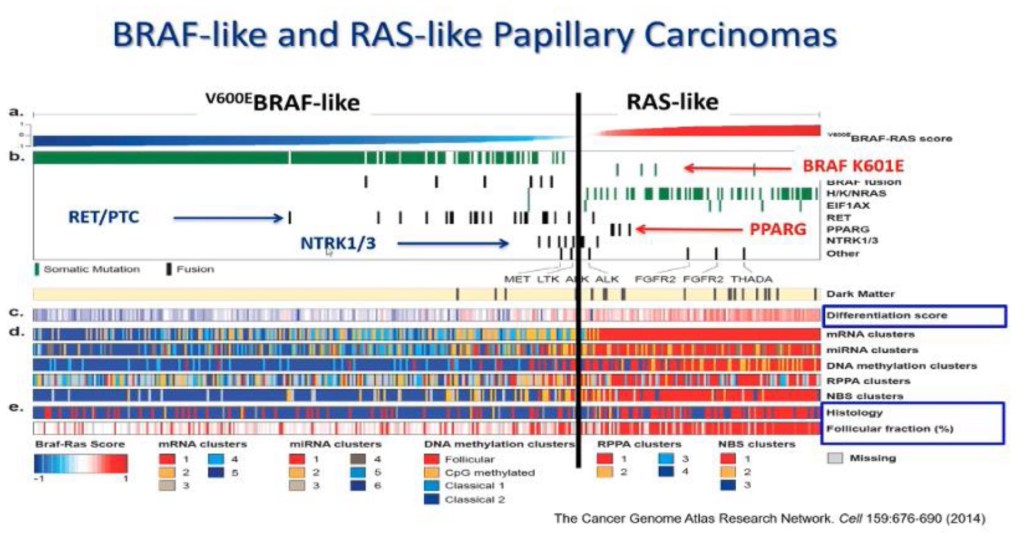
Molecular Subtypes of Papillary Thyroid Carcinomas
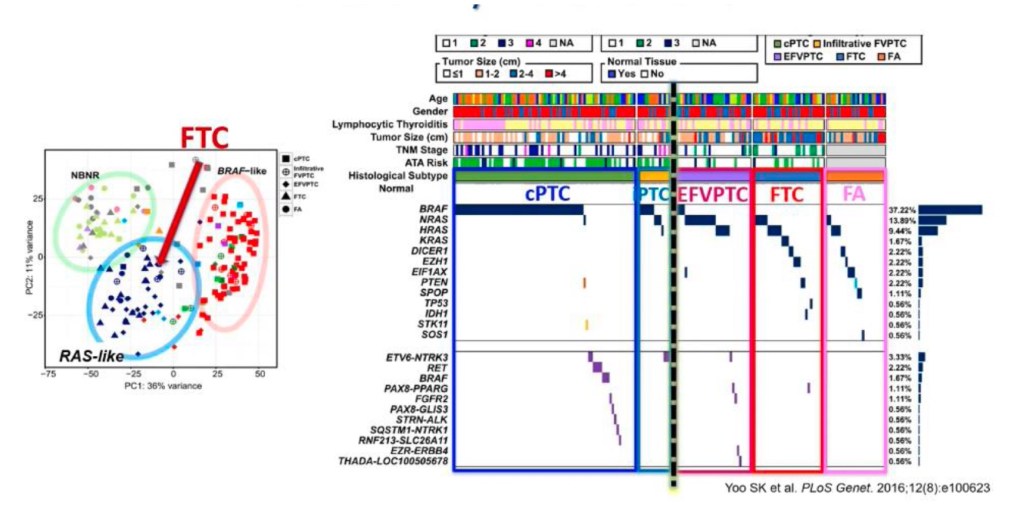
Graph exemplifying the difference between follicular pattern RAS-like tumors and classic PTC,
which is the infiltrative growth pattern.
