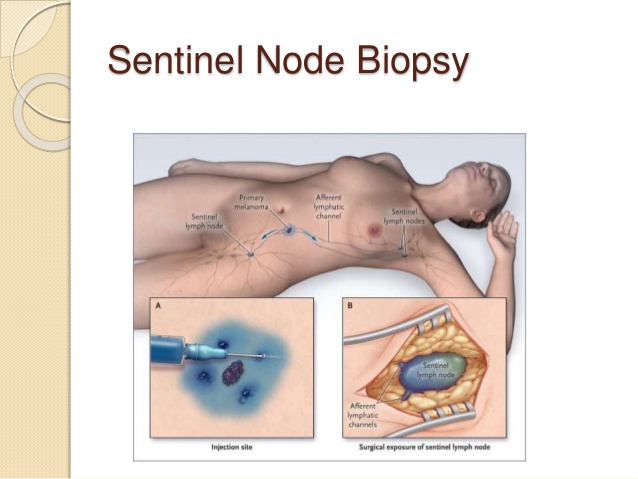
- Management of the axilla in clinically node-positive (cN1) patients following neoadjuvant chemotherapy (NAC) has evolved significantly over the past several years:
- Historically:
- The use of sentinel lymph node biopsy (SLNB) in initially node-positive patients after NAC was avoided because available data demonstrated :
- False-negative rates approaching 25%:
- Therefore, all patients were treated with axillary lymph node dissection post-NAC regardless of response to treatment.
- False-negative rates approaching 25%:
- The use of sentinel lymph node biopsy (SLNB) in initially node-positive patients after NAC was avoided because available data demonstrated :
- Three recently published prospective studies evaluated the accuracy of sentinel node biopsy following NAC in patients presenting with node-positive disease:
- Among 525 patients enrolled in the ACOSOG Z1071 trial with at least two sentinel nodes removed:
- The false-negative rate was 12.6%>
- However:
- The false-negative rate was reduced to:
- 10.8% when the SNB was performed with dual mapping (radioactive tracer and blue dye)
- 9.1% when 3 or more sentinel nodes were removed.
- The false-negative rate was reduced to:
- However:
- The false-negative rate was 12.6%>
- Similarly, the overall false-negative rate among 226 clinically node-positive patients enrolled in the SENTinel NeoAdjuvant (SENTINA) prospective study:
- Was 14.2%:
- Which decreased to 8.6% with the use of dual mapping
- 7.3% when 3 or more sentinel nodes were retrieved.
- Was 14.2%:
- Although a false-negative rate of less than 10% is acceptable in the adjuvant setting:
- Additional methods to decrease false-negative rates in this population are of clinical interest:
- As the long-term consequences of leaving behind potentially “chemotherapy-resistant” cells are unknown:
- One method to potentially improve the accuracy of SNB in this setting is:
- To clip the cN1 positive node:
- Then post-NAC localize the node and ensure removal of the clipped positive node at the time of SNB.
- In a subset analysis of patients enrolled in the ACOSOG Z1071 trial with cN1 disease and at least two sentinel nodes removed, the false-negative rate of sentinel node biopsy was 6.8% when the clipped node was one of the sentinel nodes, 19% when the clipped node was found in the axillary dissection specimen, and identified, compared to 14.3% when the clip was placed but location was unknown.
- The false-negative rate was 13.4% when no clip was placed.
- To clip the cN1 positive node:
- One method to potentially improve the accuracy of SNB in this setting is:
- As the long-term consequences of leaving behind potentially “chemotherapy-resistant” cells are unknown:
- Caudle et al. recently reviewed their experience with SNB and retrieval of the clipped node, also known as targeted axillary dissection, in clinically node-positive patients receiving NAC:
- Among 85 patients who underwent targeted axillary dissection followed by axillary lymph node dissection, the false-negative rate was 2.0% compared to 10.6% for SNB alone in this cohort (p=0.13).
- Importantly however, the mean number of sentinel lymph nodes removed in this cohort was 2.7 and dual tracer mapping was only used in 55% of the patients.
- Further study of this technique is ongoing.
- Other methods to minimize false-negative rates include documentation of treatment effect in the node:
- Which may also serve as a surrogate that the original positive node(s) has/have been retrieved.
- Although controversy exists as to the best method to evaluate the axilla post-NAC in patients presenting with node-positive disease:
- SNB with localization of the clipped node and/or SNB with dual mapping and retrieval of 3 or more sentinel nodes are both acceptable approaches to the axilla following NAC.
- Additional methods to decrease false-negative rates in this population are of clinical interest:
- Among 525 patients enrolled in the ACOSOG Z1071 trial with at least two sentinel nodes removed:
- Historically:
REFERENCES
- Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015:20;33:258-264. http://www.ncbi.nlm.nih.gov/pubmed/25452445
- Boughey JC, Suman VJ, Mittendorf EA, et al; Alliance for Clinical Trials in Oncology. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance). Ann Surg. 2015;261:547-552. https://www.ncbi.nlm.nih.gov/pubmed/25664534
- Boughey JC, Suman VJ, Mittendorf EA, et al; Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455-1461. http://www.ncbi.nlm.nih.gov/pubmed/24101169
- Caudle AS, Yang WT, Krishnaumurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072-1078. https://www.ncbi.nlm.nih.gov/pubmed/26811528
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609-618. http://www.ncbi.nlm.nih.gov/pubmed/23683750
- Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109:1255-1263. https://www.ncbi.nlm.nih.gov/pubmed/17330229

👉Rodrigo Arrangoiz MS, MD, FACS cirujano oncology y cirujano de mamá de Sociedad Quirúrgica S.C en el America British Cowdray Medical Center en la ciudad de Mexico:
-
Es experto en el manejo del cáncer de mama.

👉Es miembro de la American Society of Breast Surgeons:

Training:
• General surgery:
• Michigan State University:
• 2004 al 2010
• Surgical Oncology / Head and Neck Surgery / Endocrine Surgery:
• Fox Chase Cancer Center (Filadelfia):
• 2010 al 2012
• Masters in Science (Clinical research for health professionals):
• Drexel University (Filadelfia):
• 2010 al 2012
• Surgical Oncology / Head and Neck Surgery / Endocrine Surgery:
• IFHNOS / Memorial Sloan Kettering Cancer Center:
• 2014 al 2016






