For patients with intraepithelial neoplasia (also known as noninvasive breast cancers) treatment with tamoxifen at a dose of only 5 mg/day for 3 years, rather than the standard 20 mg/day, can reduce recurrence at a similar rate to the higher dose, according to the results of the randomized, phase 3 TAM-01 study.
-
The regimen did not significantly worsen menopausal symptoms or major adverse events compared with placebo.
-
Tamoxifen was developed in the 1960s, but the minimal effective dose has never been established, Rodrigo Arrangoiz MS, MD, FACS
-
Intraepithelial neoplasia represents 15% to 25% of all breast cancers, and includes a heterogeneous spectrum of disorders including atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS).
-
Tamoxifen is very effective in prevention (risk reducing), but its potential side effects, including:
-
Endometrial cancer, deep vein thrombosis (DVT), and menopausal symptoms, act as a barrier to its use.
De Censi and colleagues hypothesized that a reduced dose and a shorter duration would be effective.
-
That idea was based on a 2003 study from this same Italian group, which showed that 5 mg of tamoxifen was comparable to 20 mg in reducing breast cancer proliferation, as measured by Ki-67 level.
At San Antonio Breast Cancer Symposium (SABCS) 2018, the TAM-01 study which included 500 women aged less than 75 years with intraepithelial neoplasia was presented:
-
They were randomized to receive either tamoxifen 5 mg/day for 3 years, or to placebo
-
The median follow-up in the study was 5.1 years.
-
Patients had a mean age of 54 years, and just under half in both groups were premenopausal.
-
Most of the cohort had DCIS (69% of tamoxifen patients, 70% of placebo patients), followed by ADH (20% in both groups), and LCIS (11% and 10%, respectively).
In total, there were 42 breast cancer or DCIS events:
-
There were 28 such events in the placebo group compared with 14 in the tamoxifen group, for a hazard ratio (HR) of 0.48 (95% CI, 0.26–0.92; P = .024).
-
The placebo patients had a rate of 23.9 events per 1,000 person-years, compared with 11.6 events per 1,000 person-years with tamoxifen.
-
-
There were 12 cases of contralateral breast cancer in the placebo group, compared with 3 cases in the tamoxifen group, for an HR of 0.24 (95% CI, 0.07–0.87; P = .018).
-
There was one case of endometrial cancer in the tamoxifen group, and none in the placebo patients.
-
There were four other neoplasms with tamoxifen and six with placebo.
-
Both groups had one case of DVT or pulmonary embolism, and both had two cases of coronary heart disease.
-
With 20 mg/day of tamoxifen:
-
2.7 cases of endometrial cancer and 2.4 cases of DVT/pulmonary embolism would be expected.
-
-
-
Notably, the regimen did not appear to significantly worsen menopausal symptoms.
-
There was a significant increase in the frequency of daily hot flashes (P = .05), but the absolute increase was less than one.
-
There was no difference between tamoxifen and placebo with regard to vaginal dryness or pain during intercourse (P = .57), or with regard to musculoskeletal pain/arthralgia (P = .84).
-


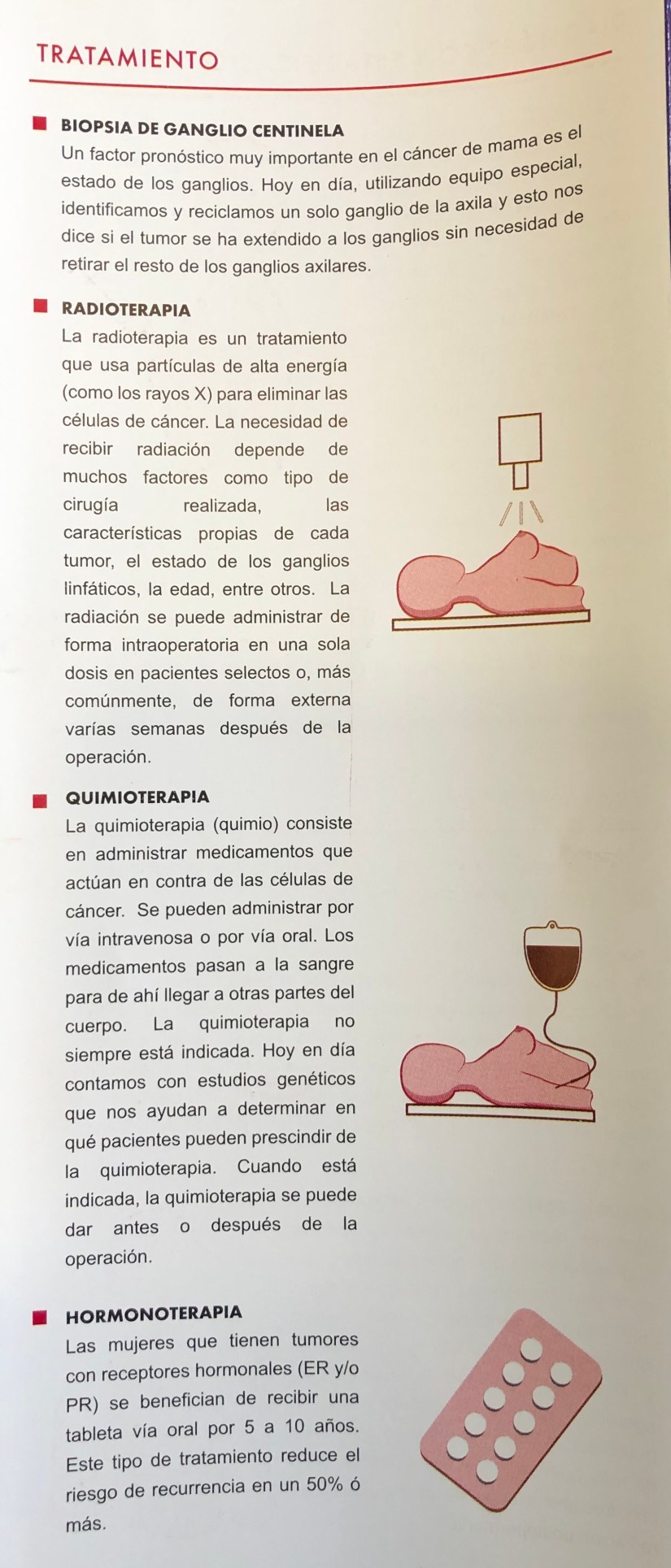



Rodrigo Arrangoiz MS, MD, FACS a surgical oncologist and is amember of Sociedad Quirúrgica S.C at the America British Cowdray Medical Center in Mexico City:
-
He is an expert in the management of breast cancer.
-
If you have any questions about risk reducing therapies for breast cancer please fill free to ask him.
-




