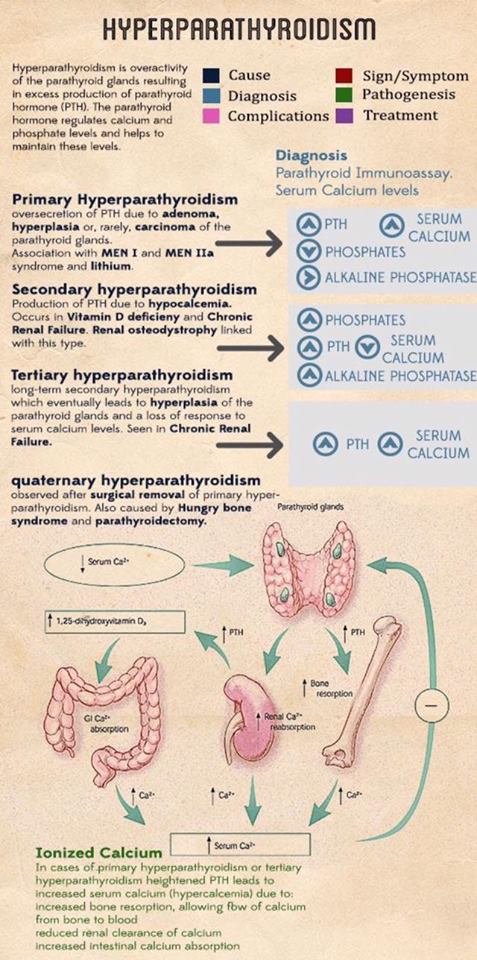
The overproduction of parathyroid hormone (PTH), termed hyperparathyroidism (HPT), can be categorized as primary, secondary, or tertiary. Primary hyperparathyroidism (PHPT) arises from an unregulated overproduction of PTH from an abnormal parathyroid gland. Increased PTH levels may also occur as a compensatory response to hypocalcemic states resulting from chronic renal failure or gastrointestinal (GI) malabsorption of calcium. This secondary HPT can be reversed by correction of the underlying problem (e.g., kidney transplantation for chronic renal failure). However, chronically stimulated parathyroid glands may occasionally become autonomous, resulting in persistence or recurrence of the hypercalcemia after successful renal transplantation, resulting in tertiary HPT.
Epidemiology and Etiology
PHPT is defined as hypercalcemia or widely fluctuating levels of serum calcium resulting from the inappropriate or autogenous secretion of PTH by one or more parathyroid glands in the absence of a known or recognized stimulus.
The most common cause of hypercalcemia in the outpatient setting is PHPT, with approximately 100,000 new cases per year reported in the United States.
Since the advent of routine laboratory testing, the prevalence of the disease has increased from 0.1% to 0.4% (one to seven cases per 1000 adults).
In a study by Yeh et al, the incidence of PHPT fluctuated between 36.3 and 120.2 cases per 100,000 women-year and 13.4 and 35.6 in 100,000 men-year. PHPT may present at any age, with the vast majority of cases occurring in patients older than 45 years of age. The mean age at diagnosis has remained between 52 and 56 years.
Women have consistently made up the preponderance of cases, with a female-to-male ratio of 3 to 4 : 1. Based on a population based study from Rochester Minnesota the higher incidence of this could be secondary (hypothetically) to estrogen deficiency after menopause that reveals underlying HPT.
The precise origin of PHPT is unknown, although exposure to low-dose therapeutic ionizing radiation and familial predisposition account for some cases.
Irradiation for acne could have accounted for a 2 to 3-fold increase in the incidence of this disease at some point in time, and a 4-fold increase was noted in survivors of the atomic bomb.
Schneider et al, in their study of 2555 patients followed for 50 years, even low doses of radiation exposure during the teenage years was associated with a slight risk of developing PHPT . In this study a dose response was documented in people receiving external-beam radiotherapy for benign diseases before their 16th birthday.
The latency period for the development of PHPT after radiation exposure is longer than that for the development of thyroid tumors, with most cases occurring 30 to 40 years after exposure.
Patients who have been radiated have similar clinical manifestations and serum calcium levels when compared to patients without a history of radiation exposure. However, the former tend to have higher PTH levels and a higher incidence of concomitant thyroid neoplasms .
Certain medications have been implicated in the development of hypercalcemia. Lithium therapy has been known to shift the set point for PTH secretion in parathyroid cells, thereby resulting in elevated PTH levels and mild hypercalcemia.
Lithium stimulates the growth of abnormal parathyroid glands in vitro and also in susceptible patients in vivo 16. Unusual metabolic features associated with lithium use include low urinary calcium excretion, normal cyclic AMP excretion and lack of calcic nephrolithiasis. The mechanism probably results from lithium linking with the calcium sensing receptor on the parathyroid glands resulting in PTH secretion.
Elevated serum calcium levels have been associated with thiazide diuretic. The overall annual age- and sex-adjusted (to 2000 U.S. whites) incidence was 7.7 (95% CI, 5.9 to 9.5) per 100,000 individuals.
The average 24-hour plasma calcium concentrations are increased with thiazide diuretic use, but the mean 24-hour PTH levels remain unchanged in subjects with normal baseline PTH levels and no evidence of hypercalciuria.
Thiazides diuretics have several metabolic effects that may contribute to increased calcium levels. A decrease in urine calcium excretion is the most likely cause, but in some cases diuretic use has been associates with a metabolic alkalosis that could also cause an increase in total serum calcium levels through a pH-dependent increase in protein-bound calcium.
Although plasma 1,25 (OH) vitamin D levels are unchanged, increased intestinal calcium absorption in response to thiazide diurectic use has been noted and could also contribute to an increase in serum calcium. One last possible explanation for the elevated serum calcium levels associated with thiazide diuretic use is hemoconcentration associated with diuresis.
Numerous genetic abnormalities have been identified in the development of PHPT, including anomalies in tumor suppressor genes and proto-oncogenes. Specific DNA mutations in a parathyroid cell may confer a proliferative advantage over normal neighboring cells, thus allowing for clonal growth. Large populations of these altered cells containing the same mutation within hyper functioning parathyroid tissue suggest that such glands are a result of clonal expansion.
The majority of PHPT cases are sporadic. Nonetheless, PHPT also occurs within the spectrum of a number of inherited disorders such as multiple endocrine neoplasia syndromes (MEN), MEN type 1 (Wermer Syndrome), MEN type 2A (Sipple Syndrome), isolated familial HPT, and familial HPT with jaw-tumor syndrome. All of these syndromes are inherited in an autosomal dominant fashion.
The earliest and most common presentation of MEN 1 is PHPT, and develops in approximately 80% to 100% of patients by age 40 years. These patients also are predisposed to the development of pancreatic neuroendocrine tumors and pituitary adenomas and, less frequently, to skin angiomas, lipomas, adrenocortical tumors, and neuroendocrine tumors of the thymus, bronchus, or stomach. MEN type 1 has been shown to result from a germline mutation in a tumor suppressor gene, called MEN1 gene, located on chromosome 11q12-13 that encodes Menin, a protein that is postulated to interact with the transcription factors JunD and nuclear factor-κB in the nucleus, in addition to replication protein A and other proteins.
Pre-symptomatic screening for mutation carriers for MEN type 1 is difficult because generally MEN1 mutations result in a nonfunctional protein and are scattered throughout the translated nine exons of the gene. MEN1 mutations also have been found in kindred’s initially suspected to represent isolated familial HPT. Screening for mutation carriers for MEN type 1 has a very high detection rate, greater than 94%, and is used in Sweden for patients with PHPT with a first-degree relative with a major endocrine tumor, age of onset is less than 30 years and/or if multiple pancreatic tumors / parathyroid hyperplasia is detected; thus these patients should be screened for MEN1 mutations.
Approximately 20% of patients with MEN type 2A (Sipple Syndrome) develop PHPT which is usually less severe. MEN type 2A is caused by a germline mutation of the RET proto-oncogene located on chromosome 10. Genotype and phenotype correlations have been noted in this syndrome in that individuals with mutations at codon 634 are more likely to develop PHPT. Patients with the familial HPT with jaw-tumor syndrome have an increased predisposition to parathyroid carcinoma. This syndrome maps to a tumor suppressor locus HRPT2 (parafibromin) on chromosome 1.
Sporadic parathyroid adenomas and some hyperplastic parathyroid glands have loss of heterozygosity (LOH) at 11q13, the site of the MEN1 gene in approximately 25% to 40% of the cases. Overexpression of PRAD1, which encodes cyclin D1, a cell cycle control protein, is found approximately 18% of parathyroid adenomas 40, 41. This was proven to result from a rearrangement on chromosome 11 that places the PRAD1 gene under the control of the PTH promoter.
Other chromosomal regions deleted in parathyroid adenomas and possibly reflecting loss of tumor suppressor genes include 1p, 6q, and 15q, whereas amplified regions suggesting oncogenes have been identified at 16p and 19p .
RET mutations are unusual in sporadic parathyroid tumor. Sporadic parathyroid cancers are characterized by uniform loss of the tumor suppressor gene RB, which is involved in cell cycle regulation, and 60% have HRPT2 (CDC73) mutations. These alterations are rare in benign parathyroid tumors and may have implications for diagnosis. The p53 tumor suppressor gene is also inactivated in a subset (30%) of parathyroid carcinomas.
Single gland adenoma is the most common cause (75% to 85%), lower pole adenomas (in relation to the thyroid) are more common than are upper pole adenomas; sizes range from 1 cm to 3 cm.

The normal weight of a parathyroid gland is approximately 40 to 50 mg, and the weight of parathyroid adenomas vary between 553.7 +/- 520.5 mg (range, 66-2536). Ectopic glands can be present (4% to 16% of cases).
PHPT is caused by the enlargement of a single parathyroid gland or parathyroid adenoma in approximately 75% to 89% of the cases, multiple adenomas or hyperplasia in 15% to 25% of the cases, and parathyroid carcinoma as the cause of PHPT is extremely rare in most parts of the world (~1%) of patients. Multi-gland adenoma arises in a significant number of patients, double adenomas are seen in approximately 2% to 12% of the cases, triple adenomas in less than 1% the cases, and four adenomas or parathyroid gland hyperplasia in less than 3% to 15% of the cases.






