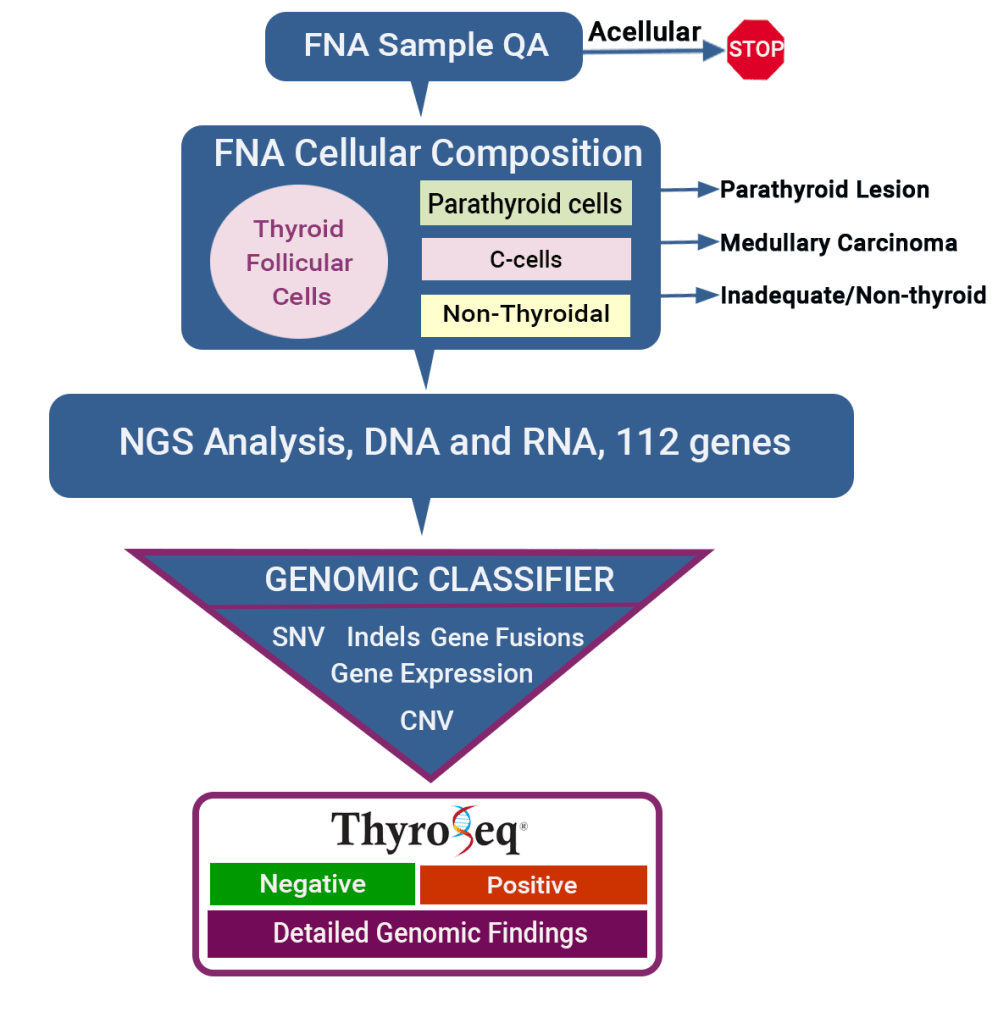
👉The primary application of ThyroSeq is to provide accurate cancer diagnosis in thyroid nodules with indeterminate FNA cytology
👉Indeterminate FNA cytology encompasses diagnostic categories III, IV, and V of the Bethesda System for Reporting Thyroid Cytopathology
👉Uncertain and variable risk of cancer in these nodules hampers clinical management of these patients
👉ThyroSeq stratifies thyroid nodules with indeterminate cytology into those that are most likely benign and can frequently be followed by observation and those that have a high probability of being cancer or pre-cancer, which in most cases need surgical management
👉ThyroSeq Clinical Validation Study Overview
- Reported in the premiere medical journal – JAMA Oncology (Steward, DL et al. JAMA Oncol. 2018.)
- Largest prospective double-blind multicenter study of any commercially available molecular test
- Highest NPV (97%) and PPV (66%) among well validated tests
- Highest reduction in diagnostic surgery:
- Allowing avoidance of surgery for up to:
- 61% of all Bethesda III / IV nodules
- 82% of indeterminate nodules with benign pathology
- Allowing avoidance of surgery for up to:
- Reliable detection of all types of thyroid tumors including Oncocytic cell cancer
- Reports probability of cancer and predicted risk of cancer recurrence, empowering individualized patient management